当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immobilization of His-tagged proteins on NiO foams for recyclable enzymatic reactors
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.apsusc.2020.147848 Pedro C. Rosado , Ricardo Meyrelles , Ana M. Macatrão , Marta C. Justino , A. Gabriela Gomes , Maria F. Montemor , Marta M. Alves , Gonçalo C. Justino , Ana P.C. Ribeiro , Karina Shimizu
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.apsusc.2020.147848 Pedro C. Rosado , Ricardo Meyrelles , Ana M. Macatrão , Marta C. Justino , A. Gabriela Gomes , Maria F. Montemor , Marta M. Alves , Gonçalo C. Justino , Ana P.C. Ribeiro , Karina Shimizu
|
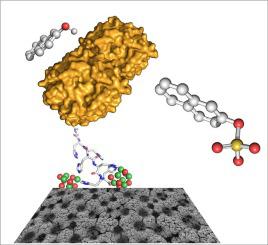
Abstract We report for the first time the use of NiO foams for specific protein binding. These affordable porous materials, with high surface area, possess the features required as supports for enzymatic catalysis. A physicochemical and computational characterization of the interaction between this support and a model His-tagged sulfotransferase was performed as a first approach to the use of NiO foams for specific protein binding. Results confirm that the His-tag binds the NiO foam and remains bound when the support is washed. The foam microporous cavities undergo marked rearrangement upon protein binding and removal, in agreement with the successful immobilization and posterior removal of the protein on the foam. Computational results suggest that the His-tag prefers a stretched conformation, acting as a bidentate ligand. Enzymatic activity of the immobilized protein was confirmed, as well as the ability to recycle the support. These results demonstrate that NiO foams constitute an affordable and effective material for His-tag protein immobilization, with potential applications, namely protein isolation and enzymatic reactor applications. The ultimate aim of this work is to contribute to a better understanding of the kinetic activity at surface level, as well as the comparison between the experimental results and the computational output.
中文翻译:

在 NiO 泡沫上固定 His 标签蛋白,用于可回收酶反应器
摘要 我们首次报道了使用 NiO 泡沫进行特异性蛋白质结合。这些经济实惠的多孔材料具有高表面积,具有作为酶催化载体所需的特性。对该支持物与模型 His 标记的磺基转移酶之间相互作用的物理化学和计算表征进行了作为使用 NiO 泡沫进行特定蛋白质结合的第一种方法。结果证实,His 标签与 NiO 泡沫结合,并在洗涤支持物时保持结合。泡沫微孔腔在蛋白质结合和去除后发生显着的重排,这与泡沫上蛋白质的成功固定和后部去除一致。计算结果表明,His 标签更喜欢拉伸构象,充当双齿配体。固定化蛋白质的酶活性以及再循环载体的能力得到证实。这些结果表明,NiO 泡沫构成了一种经济实惠且有效的 His 标签蛋白质固定材料,具有潜在的应用,即蛋白质分离和酶反应器应用。这项工作的最终目的是有助于更好地了解表面水平的动力学活动,以及实验结果与计算输出之间的比较。
更新日期:2021-01-01
中文翻译:

在 NiO 泡沫上固定 His 标签蛋白,用于可回收酶反应器
摘要 我们首次报道了使用 NiO 泡沫进行特异性蛋白质结合。这些经济实惠的多孔材料具有高表面积,具有作为酶催化载体所需的特性。对该支持物与模型 His 标记的磺基转移酶之间相互作用的物理化学和计算表征进行了作为使用 NiO 泡沫进行特定蛋白质结合的第一种方法。结果证实,His 标签与 NiO 泡沫结合,并在洗涤支持物时保持结合。泡沫微孔腔在蛋白质结合和去除后发生显着的重排,这与泡沫上蛋白质的成功固定和后部去除一致。计算结果表明,His 标签更喜欢拉伸构象,充当双齿配体。固定化蛋白质的酶活性以及再循环载体的能力得到证实。这些结果表明,NiO 泡沫构成了一种经济实惠且有效的 His 标签蛋白质固定材料,具有潜在的应用,即蛋白质分离和酶反应器应用。这项工作的最终目的是有助于更好地了解表面水平的动力学活动,以及实验结果与计算输出之间的比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号