当前位置:
X-MOL 学术
›
Appl. Geochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Abiotic anoxic reduction of AsO4 adsorbed Mg(II)-Al(III)/Fe(III)-CO3/SO4 Layered Double Hydroxides: Implications of As release and phase transformations
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apgeochem.2020.104765 Xiaoyang Lu , Mario Alberto Gomez , Bing Han , Ruonan Jiang , Xu Ma , Shuhua Yao , Baiyi Wang , Huixin Yu , Danni Zhang , Shaofeng Wang , Yongfeng Jia
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-11-01 , DOI: 10.1016/j.apgeochem.2020.104765 Xiaoyang Lu , Mario Alberto Gomez , Bing Han , Ruonan Jiang , Xu Ma , Shuhua Yao , Baiyi Wang , Huixin Yu , Danni Zhang , Shaofeng Wang , Yongfeng Jia
|
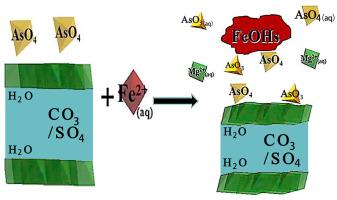
Abstract In this work, the stability of arsenate adsorbed Mg(II)–Al(III)/Fe(III)–CO3/SO4 Layered Double Hydroxides (LDHs) under three abiotic anoxic reductive conditions that may be encountered in Tailings Management Facilities (TMFs) were evaluated. At pH 8 and 10 mM Fe(II) (aq), the formation of Fe(III)-oxy/hydroxides (FeOHs) occurred for all 5 LDHs but the Fe-based LDHs precipitated the greatest amounts. All LDHs released MgAlFeCO3SO4 > MgAlCO3 > MgFeCO3/MgFeSO4. The solid surfaces remained as As(V) except for MgAlSO4, MgAlCO3, and MgAlFeCO3SO4 LDHs where 10–25% As(III) formed. Finally, at pH 10 and 0.5 mM Fe(II) (aq), the formation of FeOHs occurred to various degrees but a significant amount of CaCO3(s) precipitated. A 2-stage release and re-adsorption mechanism of total As(aq) occurred following the order: MgAlFeCO3SO4 > MgAlSO4 > MgFeCO3 > MgAlCO3 > MgFeSO4. A significant portion of the solid surfaces (30–90%) was found as As(III) for all reacted LDHs. This work provides a guideline for the environmental behavior of As(V) adsorbed LDHs where relevant underwater cover TMF abiotic reducing conditions may exist.
中文翻译:

AsO4 吸附的 Mg(II)-Al(III)/Fe(III)-CO3/SO4 层状双氢氧化物的非生物缺氧还原:As 释放和相变的影响
摘要 在这项工作中,砷酸盐吸附的 Mg(II)-Al(III)/Fe(III)-CO3/SO4 层状双氢氧化物 (LDHs) 在尾矿管理设施 (TMFs) 中可能遇到的三种非生物缺氧还原条件下的稳定性) 进行了评估。在 pH 8 和 10 mM Fe(II) (aq) 条件下,所有 5 种 LDH 均形成 Fe(III)-氧/氢氧化物 (FeOH),但 Fe 基 LDH 的沉淀量最大。所有 LDH 都释放出 MgAlFeCO3SO4 > MgAlCO3 > MgFeCO3/MgFeSO4。除了 MgAlSO4、MgAlCO3 和 MgAlFeCO3SO4 LDHs 形成 10-25% 的 As(III) 外,固体表面仍为 As(V)。最后,在 pH 10 和 0.5 mM Fe(II) (aq) 条件下,FeOH 的形成发生了不同程度,但大量 CaCO3(s) 沉淀。总 As(aq) 的 2 阶段释放和再吸附机制按照以下顺序发生:MgAlFeCO3SO4 > MgAlSO4 > MgFeCO3 > MgAlCO3 > MgFeSO4。对于所有反应的 LDH,大部分固体表面(30-90%)被发现为 As(III)。这项工作为 As(V) 吸附 LDHs 的环境行为提供了指导,其中可能存在相关的水下覆盖 TMF 非生物还原条件。
更新日期:2020-11-01
中文翻译:

AsO4 吸附的 Mg(II)-Al(III)/Fe(III)-CO3/SO4 层状双氢氧化物的非生物缺氧还原:As 释放和相变的影响
摘要 在这项工作中,砷酸盐吸附的 Mg(II)-Al(III)/Fe(III)-CO3/SO4 层状双氢氧化物 (LDHs) 在尾矿管理设施 (TMFs) 中可能遇到的三种非生物缺氧还原条件下的稳定性) 进行了评估。在 pH 8 和 10 mM Fe(II) (aq) 条件下,所有 5 种 LDH 均形成 Fe(III)-氧/氢氧化物 (FeOH),但 Fe 基 LDH 的沉淀量最大。所有 LDH 都释放出 MgAlFeCO3SO4 > MgAlCO3 > MgFeCO3/MgFeSO4。除了 MgAlSO4、MgAlCO3 和 MgAlFeCO3SO4 LDHs 形成 10-25% 的 As(III) 外,固体表面仍为 As(V)。最后,在 pH 10 和 0.5 mM Fe(II) (aq) 条件下,FeOH 的形成发生了不同程度,但大量 CaCO3(s) 沉淀。总 As(aq) 的 2 阶段释放和再吸附机制按照以下顺序发生:MgAlFeCO3SO4 > MgAlSO4 > MgFeCO3 > MgAlCO3 > MgFeSO4。对于所有反应的 LDH,大部分固体表面(30-90%)被发现为 As(III)。这项工作为 As(V) 吸附 LDHs 的环境行为提供了指导,其中可能存在相关的水下覆盖 TMF 非生物还原条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号