Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-09-19 , DOI: 10.1016/j.apsb.2020.09.010 Shaoyong Lu , Yingyi Chen , Jiacheng Wei , Mingzhu Zhao , Duan Ni , Xinheng He , Jian Zhang
|
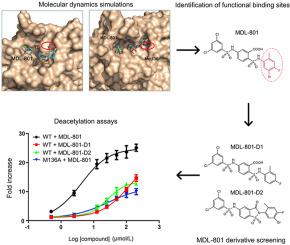
The recent discovery of activator compounds binding to an allosteric site on the NAD+-dependent protein lysine deacetylase, sirtuin 6 (SIRT6) has attracted interest and presents a pharmaceutical target for aging-related and cancer diseases. However, the mechanism underlying allosteric activation of SIRT6 by the activator MDL-801 remains largely elusive because no major conformational changes are observed upon activator binding. By combining molecular dynamics simulations with biochemical and kinetic analyses of wild-type SIRT6 and its variant M136A, we show that conformational rotation of 2-methyl-4-fluoro-5-bromo substituent on the right phenyl ring (R-ring) of MDL-801, which uncovers previously unseen hydrophobic interactions, contributes to increased activating deacetylation activity of SIRT6. This hypothesis is further supported by the two newly synthesized MDL-801 derivatives through the removal of the 5-Br atom on the R-ring (MDL-801-D1) or the restraint of the rotation of the R-ring (MDL-801-D2). We further propose that the 5-Br atom serves as an allosteric driver that controls the ligand allosteric efficacy. Our study highlights the effect of allosteric enzyme catalytic activity by activator binding and provides a rational approach for enhancing deacetylation activity.
中文翻译:

合理设计激活因子的作用揭示了SIRT6的变构激活机制
激活剂化合物与NAD +的变构位点结合的最新发现依赖蛋白的赖氨酸脱乙酰酶,sirtuin 6(SIRT6)引起了人们的兴趣,并提出了与衰老相关的疾病和癌症疾病的药物靶标。但是,激活剂MDL-801对SIRT6进行变构激活的基本机制仍然难以捉摸,因为在激活剂结合后未观察到主要构象变化。通过结合分子动力学模拟与野生型SIRT6及其变体M136A的生化和动力学分析,我们显示了MDL右苯环(R环)上的2-甲基-4-氟-5-溴取代基的构象旋转-801揭示了以前看不见的疏水性相互作用,有助于提高SIRT6的活化脱乙酰基活性。通过去除R环上的5-Br原子(MDL-801-D1)或限制R环的旋转(MDL-801),两个新合成的MDL-801衍生物进一步支持了该假设。 -D2)。我们进一步提出5-Br原子充当控制配体变构功效的变构驱动物。我们的研究通过激活剂结合突出了变构酶催化活性的影响,并提供了增强脱乙酰基活性的合理方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号