Frontiers of Chemical Science and Engineering ( IF 4.3 ) Pub Date : 2020-09-18 , DOI: 10.1007/s11705-020-1958-1 Xiaoyan Deng , Luxing Wang , Qihui Xiu , Ying Wang , Hong Han , Dongmei Dai , Yongji Xu , Hongtao Gao , Xien Liu
|
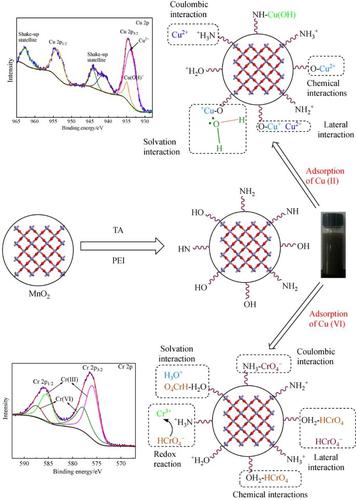
In this work, an adsorbent, which we call MnPT, was prepared by combining MnO2, polyethylenimine and tannic acid, and exhibited efficient performance for Cu(II) and Cr(VI) removal from aqueous solution. The oxygen/nitrogen-containing functional groups on the surface of MnPT might increase the enrichment of metal ions by complexation. The maximum adsorption capacities of MnPT for Cu(II) and Cr(VI) were 121.5 and 790.2 mg·g−1, respectively. The surface complexation formation model was used to elucidate the physicochemical interplay in the process of Cu(II) and Cr(VI) co-adsorption on MnPT. Electrostatic force, solvation action, adsorbate-adsorbate lateral interaction, and complexation were involved in the spontaneous adsorption process. Physical electrostatic action was dominant in the initial stage, whereas chemical action was the driving force leading to adsorption equilibrium. It should be noted that after adsorption on the surface of MnPT, Cr(VI) reacted with some reducing functional groups (hydroxylamine-NH2) and was converted into Cr(III). The adsorption capacity declined by 12% after recycling five times. Understanding the adsorption mechanism might provide a technical basis for the procedural design of heavy metal adsorbents. This MnPT nanocomposite has been proven to be a low-cost, efficient, and promising adsorbent for removing heavy metal ions from wastewater.
中文翻译:

MnO 2-聚乙烯亚胺-鞣酸复合材料对水溶液中Cu(II)和Cr(VI)的吸附性能及理化机理
在这项工作中,通过将MnO 2,聚乙烯亚胺和单宁酸混合制备了一种吸附剂,称为MnPT,在从水溶液中去除Cu(II)和Cr(VI)方面表现出高效的性能。MnPT表面的含氧/氮官能团可能通过络合增加金属离子的富集。MnPT对Cu(II)和Cr(VI)的最大吸附容量为121.5和790.2 mg·g -1, 分别。表面络合形成模型用于阐明在MnPT上Cu(II)和Cr(VI)共吸附过程中的物理化学相互作用。静电力,溶剂化作用,被吸附物-被吸附物的侧向相互作用和络合都参与自发吸附过程。在初始阶段,物理静电作用是主要的,而化学作用是导致吸附平衡的驱动力。应当指出的是,Cr(VI)吸附在MnPT表面后,与一些还原性官能团(羟胺-NH 2)并转化为Cr(III)。重复使用五次后,吸附量下降了12%。了解吸附机理可能为重金属吸附剂的程序设计提供技术基础。该MnPT纳米复合材料已被证明是一种低成本,高效且有前途的吸附剂,可从废水中去除重金属离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号