当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tutorial review on structure - dendrite growth relations in metal battery anode supports.
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-09-17 , DOI: 10.1039/d0cs00867b Wei Liu 1 , Pengcheng Liu 2 , David Mitlin 2
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-09-17 , DOI: 10.1039/d0cs00867b Wei Liu 1 , Pengcheng Liu 2 , David Mitlin 2
Affiliation
|
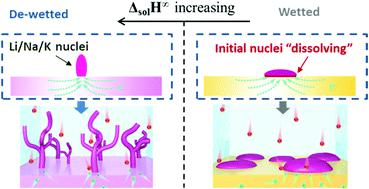
This tutorial review explains the emerging understanding of the surface and bulk chemistry – electrochemical performance relations in anode supports (aka secondary current collectors, substrates, templates, hosts) for lithium, sodium and potassium metal batteries (LMBs, SMBs or NMBs, and KMBs or PMBs). In relation to each section, the possible future research directions that may yield both new insight and improved cycling behavior are explored. Representative case studies from Li, Na and K metal anode literature are discussed. The tutorial starts with an overview of the solid electrolyte interphase (SEI), covering both the “classic” understanding of the SEI structure and the “modern” insights obtained by site-specific cryogenic stage TEM analysis. Next, the multiple roles of supports in promoting cycling stability are detailed. Without an optimized support architecture, the metal–electrolyte interface becomes geometrically unstable at a lower current density and cycle number. Taking into consideration the available literature on LMBs, SMBs and KMBs, it is concluded that effective architectures are geometrically complex and electrochemically lithiophilic, sodiophilic or potassiophilic, so as to promote conformal electrochemical wetting of the metal during plating/stripping. One way that philicity is achieved is through support oxygen surface chemistry, which yields a reversibly reactive metal–support interface. Examples of this include the well-known oxygen–carbon moieties in reduced graphene oxide (rGO), as well as classic ion battery reversible conversion reaction oxides such as SnO2. Unreactive surfaces lead to dewetted island growth of the metal, which is a precursor to dendrites, and possibly to non-uniform dissolution. Surveying the literature on various Li, Na and K metal supports, it is concluded that the key bulk thermodynamic property that will predict electrochemical wetting behavior is the enthalpy of infinite solution (ΔsolH∞) of the metal (solute) into the support (solvent). Large and negative ΔsolH∞ promotes uniform metal wetting on the support surface, corresponding to relatively low plating overpotential. Positive ΔsolH∞ promotes dewetted islands and a relatively high overpotential. This simple rule explains a broad range of studies on Li, Na and K metal – support interactions, including the previously reported correlation between mutual solubility and wetting.
中文翻译:

关于金属电池阳极支架中结构-枝晶生长关系的教程复习。
本教程的评论解释了表面和散装化学的新兴理解-在阳极支撑的电化学性能关系(又名锂,钠和钾金属电池(LMB,SMB或NMB以及KMB或PMB)的二次集电器,基底,模板,主体)。关于每个部分,探讨了可能产生新见解和改善自行车行为的未来可能的研究方向。讨论了来自Li,Na和K金属阳极文献的代表性案例研究。本教程首先概述了固体电解质中间相(SEI),涵盖了对SEI结构的“经典”理解和通过现场特定的低温阶段TEM分析获得的“现代”见解。接下来,详述了支撑物在促进循环稳定性中的多种作用。如果没有优化的支撑架构,则金属-电解质界面在较低的电流密度和循环次数下会变得几何形状不稳定。考虑到有关LMB,SMB和KMB的现有文献,可以得出结论,有效的结构在几何上是复杂的,并且具有电化学亲锂性,嗜亲性或钾亲性,从而在电镀/剥离过程中促进金属的保形电化学润湿。实现亲和性的一种方法是通过载体氧表面化学作用,产生可逆的反应性金属-载体界面。这方面的例子包括还原的氧化石墨烯(rGO)中众所周知的氧碳部分,以及经典的离子电池可逆转化反应氧化物,例如SnO 从而在电镀/剥离过程中促进金属的共形电化学润湿。实现亲和性的一种方法是通过载体氧表面化学作用,产生可逆的反应性金属-载体界面。这方面的例子包括还原的氧化石墨烯(rGO)中众所周知的氧碳部分,以及经典的离子电池可逆转化反应氧化物,例如SnO 从而在电镀/剥离过程中促进金属的共形电化学润湿。实现亲和性的一种方法是通过载体氧表面化学作用,产生可逆的反应性金属-载体界面。这方面的例子包括还原的氧化石墨烯(rGO)中众所周知的氧碳部分,以及经典的离子电池可逆转化反应氧化物,例如SnO2。未反应的表面会导致金属的露珠状岛生长,这是枝晶的前体,并可能导致不均匀溶解。测量各种锂,Na和K的金属载体的文献,可以得出结论,将预测电化学润湿行为的关键散装热力学性质是无限的溶液(Δ的焓溶胶ħ ∞)的金属(溶质)放入支承件(溶剂)。大和负Δ溶胶ħ ∞促进均匀的金属润湿在载体表面上,对应于相对低的超电势电镀。正Δ溶胶^ h ∞促进湿润的岛屿和相对较高的超潜力。这个简单的规则解释了有关Li,Na和K金属-载体相互作用的广泛研究,包括先前报道的互溶性和润湿性之间的相关性。
更新日期:2020-10-19
中文翻译:

关于金属电池阳极支架中结构-枝晶生长关系的教程复习。
本教程的评论解释了表面和散装化学的新兴理解-在阳极支撑的电化学性能关系(又名锂,钠和钾金属电池(LMB,SMB或NMB以及KMB或PMB)的二次集电器,基底,模板,主体)。关于每个部分,探讨了可能产生新见解和改善自行车行为的未来可能的研究方向。讨论了来自Li,Na和K金属阳极文献的代表性案例研究。本教程首先概述了固体电解质中间相(SEI),涵盖了对SEI结构的“经典”理解和通过现场特定的低温阶段TEM分析获得的“现代”见解。接下来,详述了支撑物在促进循环稳定性中的多种作用。如果没有优化的支撑架构,则金属-电解质界面在较低的电流密度和循环次数下会变得几何形状不稳定。考虑到有关LMB,SMB和KMB的现有文献,可以得出结论,有效的结构在几何上是复杂的,并且具有电化学亲锂性,嗜亲性或钾亲性,从而在电镀/剥离过程中促进金属的保形电化学润湿。实现亲和性的一种方法是通过载体氧表面化学作用,产生可逆的反应性金属-载体界面。这方面的例子包括还原的氧化石墨烯(rGO)中众所周知的氧碳部分,以及经典的离子电池可逆转化反应氧化物,例如SnO 从而在电镀/剥离过程中促进金属的共形电化学润湿。实现亲和性的一种方法是通过载体氧表面化学作用,产生可逆的反应性金属-载体界面。这方面的例子包括还原的氧化石墨烯(rGO)中众所周知的氧碳部分,以及经典的离子电池可逆转化反应氧化物,例如SnO 从而在电镀/剥离过程中促进金属的共形电化学润湿。实现亲和性的一种方法是通过载体氧表面化学作用,产生可逆的反应性金属-载体界面。这方面的例子包括还原的氧化石墨烯(rGO)中众所周知的氧碳部分,以及经典的离子电池可逆转化反应氧化物,例如SnO2。未反应的表面会导致金属的露珠状岛生长,这是枝晶的前体,并可能导致不均匀溶解。测量各种锂,Na和K的金属载体的文献,可以得出结论,将预测电化学润湿行为的关键散装热力学性质是无限的溶液(Δ的焓溶胶ħ ∞)的金属(溶质)放入支承件(溶剂)。大和负Δ溶胶ħ ∞促进均匀的金属润湿在载体表面上,对应于相对低的超电势电镀。正Δ溶胶^ h ∞促进湿润的岛屿和相对较高的超潜力。这个简单的规则解释了有关Li,Na和K金属-载体相互作用的广泛研究,包括先前报道的互溶性和润湿性之间的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号