当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Defluorosilylation of trifluoromethane: upgrading an environmentally damaging fluorocarbon
Chemical Communications ( IF 4.3 ) Pub Date : 2020-09-17 , DOI: 10.1039/d0cc04592f Daniel J. Sheldon 1, 2, 3, 4, 5 , Greg Coates 1, 2, 3, 4, 5 , Mark R. Crimmin 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2020-09-17 , DOI: 10.1039/d0cc04592f Daniel J. Sheldon 1, 2, 3, 4, 5 , Greg Coates 1, 2, 3, 4, 5 , Mark R. Crimmin 1, 2, 3, 4, 5
Affiliation
|
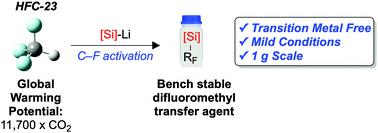
The rapid, room-temperature defluorosilylation of trifluoromethane, a highly potent greenhouse gas, has been achieved using a simple silyl lithium reagent. An extensive computational mechanistic analysis provides a viable reaction pathway and demonstrates the unexpected electrophilic nature of LiCF3. The reaction generates a bench stable fluorinated building block that shows promise as an easy-to-use difluoromethylating agent. The difluoromethyl group is an increasingly important bioisostere in active pharmaceutical ingredients, and therefore our methodology creates value from waste. The potential scalability of the process has been demonstrated by achieving the reaction on a gram-scale.
中文翻译:

三氟甲烷的脱氟硅烷化:升级对环境有害的碳氟化合物
使用简单的甲硅烷基锂试剂即可实现室温下三氟甲烷(一种强效温室气体)的快速,室温脱氟甲硅烷基化。广泛的计算机理分析提供了可行的反应途径,并证明了LiCF 3出乎意料的亲电子性质。该反应生成了一个台式稳定的氟化结构单元,显示出有望成为易于使用的二氟甲基化剂。在活性药物成分中,二氟甲基是越来越重要的生物等排体,因此我们的方法从废物中创造价值。该过程的潜在可扩展性已通过在克级上实现反应得到了证明。
更新日期:2020-09-25
中文翻译:

三氟甲烷的脱氟硅烷化:升级对环境有害的碳氟化合物
使用简单的甲硅烷基锂试剂即可实现室温下三氟甲烷(一种强效温室气体)的快速,室温脱氟甲硅烷基化。广泛的计算机理分析提供了可行的反应途径,并证明了LiCF 3出乎意料的亲电子性质。该反应生成了一个台式稳定的氟化结构单元,显示出有望成为易于使用的二氟甲基化剂。在活性药物成分中,二氟甲基是越来越重要的生物等排体,因此我们的方法从废物中创造价值。该过程的潜在可扩展性已通过在克级上实现反应得到了证明。











































 京公网安备 11010802027423号
京公网安备 11010802027423号