当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Nabscessin A from Inositol and D-Camphor.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.joc.0c01839 Hsin-Ying Wu,Wei-Yi Wang,Chia-Chi Feng,Duen-Ren Hou
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.joc.0c01839 Hsin-Ying Wu,Wei-Yi Wang,Chia-Chi Feng,Duen-Ren Hou
|
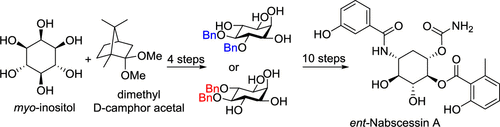
An enantiomer of nabscessin A (1), an aminocyclitol amide with antimicrobial activity, was synthesized from myo-inositol and dimethyl d-camphor acetal in 14 steps. Formal synthesis of natural nabscessin A was also achieved through the new approach to access both enantiomers of 4,5-di-O-benzyl-myo-inositol, derived from the same set of starting materials. This synthesis features utilizations of the existing framework of myo-inositol and a regioselective esterification.
中文翻译:

从肌醇和D-樟脑不对称合成纳豆素A。
nabscessin A的对映体(1),一个氨基环醇酰胺具有抗微生物活性,由合成肌醇肌醇和二甲基d -樟脑缩醛在14个步骤。还可以通过新方法来获得天然那巴豆素A的形式合成,该新方法可访问衍生自同一组起始原料的4,5-二-O-苄基-肌醇肌醇的两种对映体。该合成的特征在于利用了肌醇的现有构架和区域选择性酯化。
更新日期:2020-10-17
中文翻译:

从肌醇和D-樟脑不对称合成纳豆素A。
nabscessin A的对映体(1),一个氨基环醇酰胺具有抗微生物活性,由合成肌醇肌醇和二甲基d -樟脑缩醛在14个步骤。还可以通过新方法来获得天然那巴豆素A的形式合成,该新方法可访问衍生自同一组起始原料的4,5-二-O-苄基-肌醇肌醇的两种对映体。该合成的特征在于利用了肌醇的现有构架和区域选择性酯化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号