当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pathway Engineering of Anthracyclines: Blazing Trails in Natural Product Glycodiversification.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.joc.0c01863 Katelyn V Brown 1 , Benjamin Nji Wandi 2 , Mikko Metsä-Ketelä 2 , S Eric Nybo 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.joc.0c01863 Katelyn V Brown 1 , Benjamin Nji Wandi 2 , Mikko Metsä-Ketelä 2 , S Eric Nybo 1
Affiliation
|
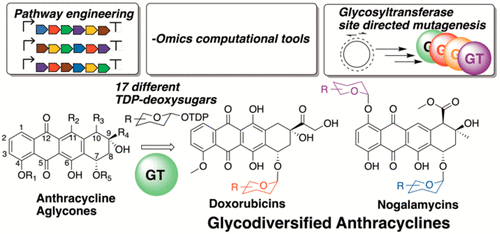
The anthracyclines are structurally diverse anticancer natural products that bind to DNA and poison the topoisomerase II–DNA complex in cancer cells. Rational modifications in the deoxysugar functionality are especially advantageous for synthesizing drugs with improved potency. Combinatorial biosynthesis of glycosyltransferases and deoxysugar synthesis enzymes is indispensable for the generation of glycodiversified anthracyclines. This Synopsis considers recent advances in glycosyltransferase structural biology and site-directed mutagenesis, pathway engineering, and deoxysugar combinatorial biosynthesis with a focus on the generation of “new-to-nature” anthracycline analogues.
中文翻译:

蒽环类药物通路工程:天然产物糖基多样化的新路径。
蒽环类化合物是结构多样的抗癌天然产物,可与 DNA 结合并毒害癌细胞中的拓扑异构酶 II-DNA 复合物。脱氧糖官能团的合理修饰对于合成具有改进效力的药物尤其有利。糖基转移酶和脱氧糖合成酶的组合生物合成对于生成糖基多样化的蒽环类药物是必不可少的。本概要介绍了糖基转移酶结构生物学和定点诱变、通路工程和脱氧糖组合生物合成方面的最新进展,重点是“新天然”蒽环类类似物的产生。
更新日期:2020-10-02
中文翻译:

蒽环类药物通路工程:天然产物糖基多样化的新路径。
蒽环类化合物是结构多样的抗癌天然产物,可与 DNA 结合并毒害癌细胞中的拓扑异构酶 II-DNA 复合物。脱氧糖官能团的合理修饰对于合成具有改进效力的药物尤其有利。糖基转移酶和脱氧糖合成酶的组合生物合成对于生成糖基多样化的蒽环类药物是必不可少的。本概要介绍了糖基转移酶结构生物学和定点诱变、通路工程和脱氧糖组合生物合成方面的最新进展,重点是“新天然”蒽环类类似物的产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号