当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Manganese-Catalyzed Oxidative Azidation of C(sp3)-H Bonds under Electrophotocatalytic Conditions
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-17 , DOI: 10.1021/jacs.0c08437 Linbin Niu 1 , Chongyu Jiang 1 , Yuwei Liang 1 , Dingdong Liu 1 , Faxiang Bu 1 , Renyi Shi 1 , Hong Chen 1 , Abhishek Dutta Chowdhury 1 , Aiwen Lei 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-09-17 , DOI: 10.1021/jacs.0c08437 Linbin Niu 1 , Chongyu Jiang 1 , Yuwei Liang 1 , Dingdong Liu 1 , Faxiang Bu 1 , Renyi Shi 1 , Hong Chen 1 , Abhishek Dutta Chowdhury 1 , Aiwen Lei 1, 2
Affiliation
|
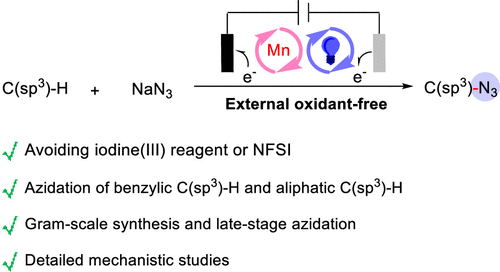
The selective installation of azide groups into C(sp3)-H bonds is a priority research topic in organic synthesis, particularly in pharma-ceutical discovery and late-stage diversification. Herein, we demon-strate a generalized manganese-catalyzed oxidative azidation meth-odology of C(sp3)-H bonds using nucleophilic NaN3 as an azide source under electrophotocatalytic conditions. This approach allows us to perform the reaction without the necessity of adding an excess of the substrate and successfully avoiding the use of stoichiometric chemical oxidants such as iodine(III) reagent or NFSI. A series of tertiary and secondary benzylic C(sp3)-H, aliphatic C(sp3)-H, and drug-molecules-based C(sp3)-H bonds containing in substrates are well tolerated under our protocol. The simultaneous gram-scale synthesis and the ease of transformation of azide to amine collec-tively advocate for the potential application in the preparative syn-thesis. Good reactivity of tertiary benzylic C(sp3)-H bond and se-lectivity of tertiary aliphatic C(sp3)-H bond containing in substrates to incorporate nitrogen-based functionality at tertiary alkyl group also provide opportunities to manipulate numerous potential medic-inal candidates. We anticipate our synthetic protocol, consisting of metal catalysis, electrochemistry and photochemistry, would pro-vide a new sustainable option to execute challenging organic syn-thetic transformations.
中文翻译:

电光催化条件下锰催化的 C(sp3)-H 键氧化叠氮化
将叠氮基选择性安装到 C(sp3)-H 键中是有机合成中的优先研究课题,特别是在药物发现和后期多样化中。在此,我们展示了在电光催化条件下使用亲核 NaN3 作为叠氮化物源的 C(sp3)-H 键的广义锰催化氧化叠氮化方法。这种方法使我们能够在无需添加过量底物的情况下进行反应,并成功避免使用化学计量化学氧化剂,如碘 (III) 试剂或 NFSI。在我们的协议下,基质中包含的一系列三级和二级苄基 C(sp3)-H、脂肪族 C(sp3)-H 和基于药物分子的 C(sp3)-H 键具有良好的耐受性。同时克级规模合成和叠氮化物易于转化为胺共同提倡在制备合成中的潜在应用。叔苄基 C(sp3)-H 键的良好反应性和底物中含有的叔脂肪族 C(sp3)-H 键的选择性以在叔烷基上结合基于氮的官能团也提供了操纵许多潜在药物候选物的机会. 我们预计我们的合成方案,包括金属催化、电化学和光化学,将为执行具有挑战性的有机合成转化提供一个新的可持续选择。叔苄基 C(sp3)-H 键的良好反应性和底物中含有的叔脂肪族 C(sp3)-H 键的选择性以在叔烷基上结合基于氮的官能团也提供了操纵许多潜在药物候选物的机会. 我们预计我们的合成方案,包括金属催化、电化学和光化学,将为执行具有挑战性的有机合成转化提供一个新的可持续选择。叔苄基 C(sp3)-H 键的良好反应性和底物中含有的叔脂肪族 C(sp3)-H 键的选择性以在叔烷基上结合基于氮的官能团也提供了操纵许多潜在药物候选物的机会. 我们预计我们的合成方案,包括金属催化、电化学和光化学,将为执行具有挑战性的有机合成转化提供一个新的可持续选择。
更新日期:2020-09-17
中文翻译:

电光催化条件下锰催化的 C(sp3)-H 键氧化叠氮化
将叠氮基选择性安装到 C(sp3)-H 键中是有机合成中的优先研究课题,特别是在药物发现和后期多样化中。在此,我们展示了在电光催化条件下使用亲核 NaN3 作为叠氮化物源的 C(sp3)-H 键的广义锰催化氧化叠氮化方法。这种方法使我们能够在无需添加过量底物的情况下进行反应,并成功避免使用化学计量化学氧化剂,如碘 (III) 试剂或 NFSI。在我们的协议下,基质中包含的一系列三级和二级苄基 C(sp3)-H、脂肪族 C(sp3)-H 和基于药物分子的 C(sp3)-H 键具有良好的耐受性。同时克级规模合成和叠氮化物易于转化为胺共同提倡在制备合成中的潜在应用。叔苄基 C(sp3)-H 键的良好反应性和底物中含有的叔脂肪族 C(sp3)-H 键的选择性以在叔烷基上结合基于氮的官能团也提供了操纵许多潜在药物候选物的机会. 我们预计我们的合成方案,包括金属催化、电化学和光化学,将为执行具有挑战性的有机合成转化提供一个新的可持续选择。叔苄基 C(sp3)-H 键的良好反应性和底物中含有的叔脂肪族 C(sp3)-H 键的选择性以在叔烷基上结合基于氮的官能团也提供了操纵许多潜在药物候选物的机会. 我们预计我们的合成方案,包括金属催化、电化学和光化学,将为执行具有挑战性的有机合成转化提供一个新的可持续选择。叔苄基 C(sp3)-H 键的良好反应性和底物中含有的叔脂肪族 C(sp3)-H 键的选择性以在叔烷基上结合基于氮的官能团也提供了操纵许多潜在药物候选物的机会. 我们预计我们的合成方案,包括金属催化、电化学和光化学,将为执行具有挑战性的有机合成转化提供一个新的可持续选择。



























 京公网安备 11010802027423号
京公网安备 11010802027423号