当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lycosquarrines A-R, Lycopodium Alkaloids from Phlegmariurus squarrosus.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.jnatprod.9b00815 Xinliu Zhu 1 , Dan Xia 1 , Zhongbo Zhou 2 , Saisai Xie 3 , Zehui Shi 1 , Guimin Chen 1 , Lulu Wang 1 , Ke Pan 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.jnatprod.9b00815 Xinliu Zhu 1 , Dan Xia 1 , Zhongbo Zhou 2 , Saisai Xie 3 , Zehui Shi 1 , Guimin Chen 1 , Lulu Wang 1 , Ke Pan 1
Affiliation
|
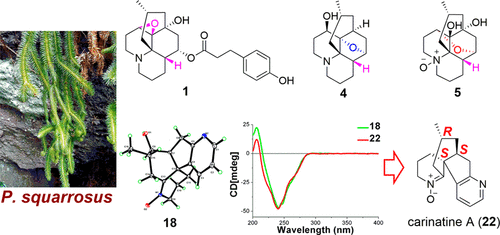
Eighteen new Lycopodium alkaloids, lycosquarrines A–R (1–18), and eight known alkaloids were isolated from the aerial parts of Phlegmariurus squarrosus. Compounds 1–5 and 19, identified from natural sources for the first time, are uncommon lycopodine-type alkaloids with β-oriented H-4. Pentacyclic 4 and 5 represent the first examples of 5,12- and 5,11-epoxy Lycopodium alkaloids, respectively, and an epoxide-opening cyclization reaction is suggested to be a key step in their biosynthesis. Compound 18 possesses the same carbon skeleton as carinatine A (22), which was previously reported as a unique Lycopodium alkaloid with a 5/6/6/6 ring system. X-ray crystallographic data analysis was used to determine the absolute configuration of 18, leading to the establishment of the absolute configuration of 22 by comparison of the ECD spectra. An anti-acetylcholinesterase activity assay showed that 11 and 20 exhibited inhibitory activities with IC50 values of 4.2 and 2.1 μM, respectively.
中文翻译:

Lycosquarrines AR,来自 Phlegmariurus squarrosus 的 Lycopodium 生物碱。
十八新石松生物碱,lycosquarrines A-R(1 - 18),和8种已知生物碱从地上部分分离马尾squarrosus。化合物1 - 5和19,从首次天然来源鉴定,是罕见的lycopodine型生物碱与面向β-H-4。五环4和5 分别代表 5,12- 和 5,11- 环氧石松生物碱的第一个例子,环氧化物开环反应被认为是它们生物合成的关键步骤。化合物18具有与carinatine A相同的碳骨架(22),之前报道为具有 5/6/6/6 环系统的独特石松生物碱。X 射线晶体学数据分析用于确定18的绝对构型,从而通过比较 ECD 光谱确定22的绝对构型。抗乙酰胆碱酯酶活性测定表明,11和20表现出抑制活性,IC 50值分别为 4.2 和 2.1 μM。
更新日期:2020-10-26
中文翻译:

Lycosquarrines AR,来自 Phlegmariurus squarrosus 的 Lycopodium 生物碱。
十八新石松生物碱,lycosquarrines A-R(1 - 18),和8种已知生物碱从地上部分分离马尾squarrosus。化合物1 - 5和19,从首次天然来源鉴定,是罕见的lycopodine型生物碱与面向β-H-4。五环4和5 分别代表 5,12- 和 5,11- 环氧石松生物碱的第一个例子,环氧化物开环反应被认为是它们生物合成的关键步骤。化合物18具有与carinatine A相同的碳骨架(22),之前报道为具有 5/6/6/6 环系统的独特石松生物碱。X 射线晶体学数据分析用于确定18的绝对构型,从而通过比较 ECD 光谱确定22的绝对构型。抗乙酰胆碱酯酶活性测定表明,11和20表现出抑制活性,IC 50值分别为 4.2 和 2.1 μM。











































 京公网安备 11010802027423号
京公网安备 11010802027423号