当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility Enhancement of Hydrophobic Substances in Water/Cyrene Mixtures: A Computational Study
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.iecr.0c03155 Dinis O. Abranches 1 , Jordana Benfica 1 , Seishi Shimizu 2 , João A. P. Coutinho 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.iecr.0c03155 Dinis O. Abranches 1 , Jordana Benfica 1 , Seishi Shimizu 2 , João A. P. Coutinho 1
Affiliation
|
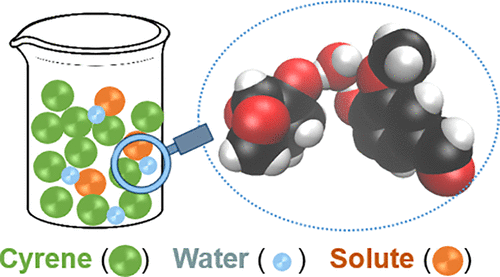
Cyrene, a recently introduced biobased green solvent, behaves as a hydrotrope, greatly increasing the aqueous solubility of hydrophobic substances in water. Moreover, water, when present in small quantities, has also been shown to increase the solubility of hydrophobic substances in Cyrene. In spite of large practical importance, the understanding of the solvation ability of water/Cyrene mixtures is hampered by a lack of fundamental knowledge of their solvation mechanisms, which is further complicated by a chemical equilibrium established between the ketone form of Cyrene and water, yielding the diol form of Cyrene. In this work, the conductor-like screening model for real solvents (COSMO-RS) is used to rationalize the cosolvency effect of water on Cyrene. It is shown here that COSMO-RS can accurately predict the solubility of hydrophobic substances in water/Cyrene mixtures in the entire composition range from pure water to pure Cyrene. The predictions of the model show that the ketone form of Cyrene controls its hydrotropic ability, supporting the concept of cooperative hydrotropy. Using COSMO-RS to assess molecular contact probability, it is also shown that water, when added in small quantities to Cyrene, acts as a cosolvent by preferentially interacting with the solute as a hydrogen bond acceptor, effectively doubling the hydrogen-bond donning ability of the solute and, thus, maximizing the interactions with Cyrene. In turn, this leads to a solubility increase of hydrophobic substances in wet Cyrene.
中文翻译:

水/ C混合物中疏水性物质的溶解度增强:计算研究
yr是一种最近引入的生物基绿色溶剂,可充当水溶助长剂,大大提高了疏水性物质在水中的水溶性。此外,当水少量存在时,也已显示出其可增加疏水性物质在C中的溶解度。尽管具有很大的实际重要性,但由于缺乏对它们的溶剂化机理的基本了解,因此对水/,混合物的溶剂化能力的理解受到了阻碍,而酮和水的酮形式之间建立了化学平衡,这使得制备过程更加复杂。 C的二醇形式。在这项工作中,使用针对真实溶剂的类似导体的筛选模型(COSMO-RS)来合理化水对C的共溶作用。此处显示,COSMO-RS可以准确预测疏水性物质在纯净水到纯净乙炔的整个组成范围内在水/乙炔混合物中的溶解度。该模型的预测表明,C的酮形式控制了其水溶能力,从而支持了协同水溶的概念。使用COSMO-RS评估分子的接触概率,还表明,将水少量添加到Cyrene中后,它会与作为氢键受体的溶质优先相互作用,从而作为助溶剂,有效地加倍了Cyrene的氢键赋予能力。溶质,从而最大程度地提高与Cyrene的相互作用。反过来,这导致疏水性物质在湿C中的溶解度增加。该模型的预测表明,C的酮形式控制了其水溶能力,从而支持了协同水溶的概念。使用COSMO-RS评估分子接触概率,还表明,将水少量添加到Cyrene中后,它会优先与作为氢键受体的溶质相互作用,从而有效地加倍了Cyrene的氢键赋予能力,从而作为助溶剂。溶质,从而最大程度地提高与Cyrene的相互作用。反过来,这导致疏水性物质在湿C中的溶解度增加。该模型的预测表明,C的酮形式控制了其水溶能力,从而支持了协同水溶的概念。使用COSMO-RS评估分子的接触概率,还表明,将水少量添加到Cyrene中后,它会与作为氢键受体的溶质优先相互作用,从而作为助溶剂,有效地加倍了Cyrene的氢键赋予能力。溶质,从而最大程度地提高与Cyrene的相互作用。反过来,这导致疏水性物质在湿C中的溶解度增加。通过优先与作为氢键受体的溶质相互作用,从而有效地使溶质的氢键赋予能力加倍,从而最大程度地提高与Cyrene的相互作用,从而充当助溶剂。反过来,这导致疏水性物质在湿C中的溶解度增加。通过优先与作为氢键受体的溶质相互作用,从而有效地使溶质的氢键赋予能力加倍,从而最大程度地提高与Cyrene的相互作用,从而充当助溶剂。反过来,这导致疏水性物质在湿C中的溶解度增加。
更新日期:2020-10-07
中文翻译:

水/ C混合物中疏水性物质的溶解度增强:计算研究
yr是一种最近引入的生物基绿色溶剂,可充当水溶助长剂,大大提高了疏水性物质在水中的水溶性。此外,当水少量存在时,也已显示出其可增加疏水性物质在C中的溶解度。尽管具有很大的实际重要性,但由于缺乏对它们的溶剂化机理的基本了解,因此对水/,混合物的溶剂化能力的理解受到了阻碍,而酮和水的酮形式之间建立了化学平衡,这使得制备过程更加复杂。 C的二醇形式。在这项工作中,使用针对真实溶剂的类似导体的筛选模型(COSMO-RS)来合理化水对C的共溶作用。此处显示,COSMO-RS可以准确预测疏水性物质在纯净水到纯净乙炔的整个组成范围内在水/乙炔混合物中的溶解度。该模型的预测表明,C的酮形式控制了其水溶能力,从而支持了协同水溶的概念。使用COSMO-RS评估分子的接触概率,还表明,将水少量添加到Cyrene中后,它会与作为氢键受体的溶质优先相互作用,从而作为助溶剂,有效地加倍了Cyrene的氢键赋予能力。溶质,从而最大程度地提高与Cyrene的相互作用。反过来,这导致疏水性物质在湿C中的溶解度增加。该模型的预测表明,C的酮形式控制了其水溶能力,从而支持了协同水溶的概念。使用COSMO-RS评估分子接触概率,还表明,将水少量添加到Cyrene中后,它会优先与作为氢键受体的溶质相互作用,从而有效地加倍了Cyrene的氢键赋予能力,从而作为助溶剂。溶质,从而最大程度地提高与Cyrene的相互作用。反过来,这导致疏水性物质在湿C中的溶解度增加。该模型的预测表明,C的酮形式控制了其水溶能力,从而支持了协同水溶的概念。使用COSMO-RS评估分子的接触概率,还表明,将水少量添加到Cyrene中后,它会与作为氢键受体的溶质优先相互作用,从而作为助溶剂,有效地加倍了Cyrene的氢键赋予能力。溶质,从而最大程度地提高与Cyrene的相互作用。反过来,这导致疏水性物质在湿C中的溶解度增加。通过优先与作为氢键受体的溶质相互作用,从而有效地使溶质的氢键赋予能力加倍,从而最大程度地提高与Cyrene的相互作用,从而充当助溶剂。反过来,这导致疏水性物质在湿C中的溶解度增加。通过优先与作为氢键受体的溶质相互作用,从而有效地使溶质的氢键赋予能力加倍,从而最大程度地提高与Cyrene的相互作用,从而充当助溶剂。反过来,这导致疏水性物质在湿C中的溶解度增加。









































 京公网安备 11010802027423号
京公网安备 11010802027423号