当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable Biomineralization of CdS Quantum Dots by Immobilized Cystathionine γ-Lyase
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-09-17 , DOI: 10.1021/acssuschemeng.0c04600 Nur Koncuy Ozdemir 1 , Joseph P. Cline 2 , Christopher J. Kiely 1, 2 , Steven McIntosh 1 , Mark A. Snyder 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-09-17 , DOI: 10.1021/acssuschemeng.0c04600 Nur Koncuy Ozdemir 1 , Joseph P. Cline 2 , Christopher J. Kiely 1, 2 , Steven McIntosh 1 , Mark A. Snyder 1
Affiliation
|
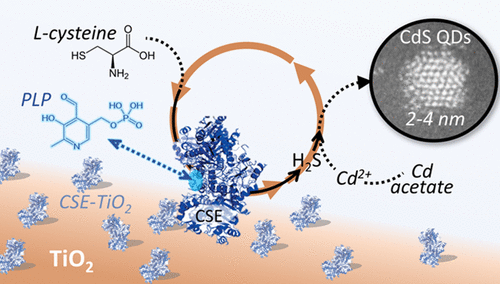
The development of scalable, green, aqueous phase, nanomaterial synthesis routes that do not sacrifice the functional performance of the materials remains a persistent challenge. Bioinspired methodologies have proven effective for batchwise synthesis of highly functional nanomaterials but require scalable strategies for the recovery and reuse of costly enzymes. Herein, we demonstrate that facile immobilization of pyridoxal phosphate (PLP)-dependent cystathionine γ-lyase (CSE) on inexpensive TiO2 supports enables the cyclic biomineralization of size-controlled nanocrystalline CdS quantum dots (QDs). When supplied with PLP to regenerate the active center, the immobilized enzyme demonstrates no more than ca. 5% activity loss over six cycles while maintaining a tight size distribution of the nanocrystals (1.72 ± 0.44 nm). This demonstrates the promise for this approach as a simple, scalable strategy for continuous single-enzyme-based biomineralization of functional nanocrystals.
中文翻译:

固定化的胱硫醚γ-裂解酶可扩展的CdS量子点生物矿化
可扩展的,绿色的,水相的,纳米材料合成路线的发展,而不会牺牲材料的功能性能,这仍然是一个持续的挑战。生物启发的方法论已被证明可用于高功能纳米材料的批量合成,但需要可扩展的策略来回收和再利用昂贵的酶。在本文中,我们证明了廉价的TiO 2上容易固定吡pyr醛磷酸盐(PLP)依赖性胱硫醚γ-裂合酶(CSE)支持物使尺寸受控的纳米晶CdS量子点(QDs)循环生物矿化。当与PLP一起提供以再生活性中心时,固定化酶的酶解活性不超过ca。在六个周期内损失了5%的活性,同时保持了纳米晶体的紧密尺寸分布(1.72±0.44 nm)。这证明了这种方法作为一种简单,可扩展的策略的前景,可用于基于功能性单晶的连续单酶生物矿化。
更新日期:2020-10-12
中文翻译:

固定化的胱硫醚γ-裂解酶可扩展的CdS量子点生物矿化
可扩展的,绿色的,水相的,纳米材料合成路线的发展,而不会牺牲材料的功能性能,这仍然是一个持续的挑战。生物启发的方法论已被证明可用于高功能纳米材料的批量合成,但需要可扩展的策略来回收和再利用昂贵的酶。在本文中,我们证明了廉价的TiO 2上容易固定吡pyr醛磷酸盐(PLP)依赖性胱硫醚γ-裂合酶(CSE)支持物使尺寸受控的纳米晶CdS量子点(QDs)循环生物矿化。当与PLP一起提供以再生活性中心时,固定化酶的酶解活性不超过ca。在六个周期内损失了5%的活性,同时保持了纳米晶体的紧密尺寸分布(1.72±0.44 nm)。这证明了这种方法作为一种简单,可扩展的策略的前景,可用于基于功能性单晶的连续单酶生物矿化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号