当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Band-Gap Engineering of FeF3·0.33H2O Nanosphere via Ni Doping as a High-Performance Lithium-Ion Battery Cathode
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-09-17 , DOI: 10.1021/acssuschemeng.0c05258 Min Liu 1 , Qun Wang 1 , Biaobing Chen 1 , Huiling Lei 1 , Lei Liu 1 , Chun Wu 2 , Xianyou Wang 1 , Zhenhua Yang 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-09-17 , DOI: 10.1021/acssuschemeng.0c05258 Min Liu 1 , Qun Wang 1 , Biaobing Chen 1 , Huiling Lei 1 , Lei Liu 1 , Chun Wu 2 , Xianyou Wang 1 , Zhenhua Yang 1
Affiliation
|
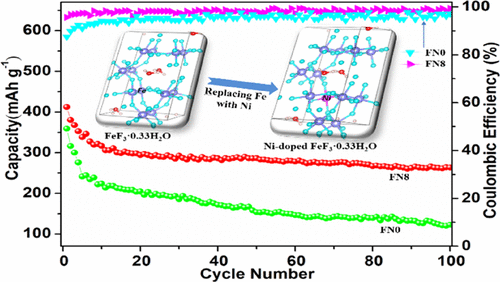
Iron-based fluorides, a kind of intercalation–conversion cathode material, are considered as one of the most promising candidate cathode materials for lithium-ion batteries due to their high capacity and cheap cost. Nevertheless, poor electrical conductivity and sluggish reaction kinetics upon cycling impede their practical application. Herein, Ni-doped FeF3·0.33H2O has been put forward and fabricated by a simple solvothermal method to overcome the above deficiency. The impact of Ni doping on the physical and electrochemical performances has been studied by first-principles calculations combined with comprehensive physicochemical characterizations. The results reveal that appropriate substitution of Fe sites by Ni can result in F vacancies without changing the intrinsic crystal structure, but it not only reduces the band gap and improves its intrinsic conductivity but also broadens the ion channel, thus facilitating the rapid transport of ions and electrons to acquire excellent electrochemical properties. Compared with the bare FeF3·0.33H2O, the appropriate amount (8%) of Ni2+ doping exhibits higher reversible capacity, longer cyclic stability, and better rate capability. It presents a 412 mAh g–1 initial discharge capacity and remains 264 mAh g–1 after 100 cycles at 0.25 C (1 C = 200 mA g–1) within a range of 1.5–4.5 V. Additionally, at 2.0 C, it still delivers a high discharge capacity of 248 mAh g–1. The excellent electrochemical performance is ascribed to the improvement of electronic conductivity and reaction kinetics caused by the moderate Ni doping.
中文翻译:

Ni掺杂作为高性能锂离子电池阴极的FeF 3 ·0.33H 2 O纳米球带隙工程
铁基氟化物是一种插层-转换阴极材料,由于其高容量和廉价的成本,被认为是锂离子电池最有希望的候选阴极材料之一。然而,循环时差的电导率和缓慢的反应动力学阻碍了它们的实际应用。此处,Ni掺杂的FeF 3 ·0.33H 2为了克服上述缺陷,已经提出并通过简单的溶剂热法制备了O。通过第一性原理计算和综合的理化特性研究了镍掺杂对物理和电化学性能的影响。结果表明,Ni适当取代Fe位点可导致F空位而不改变本征晶体结构,但不仅减小了带隙,提高了本征电导率,而且拓宽了离子通道,从而促进了离子的快速迁移。和电子获得优异的电化学性能。与裸FeF 3 ·0.33H 2 O相比,适量(8%)的Ni 2+掺杂具有更高的可逆容量,更长的循环稳定性和更好的速率能力。它具有412 mAh g –1的初始放电容量,在0.25 C(1 C = 200 mA g –1)在1.5–4.5 V的范围内经过100个循环后仍保持264 mAh g –1。此外,在2.0 C时,它仍具有仍可提供248 mAh g –1的高放电容量。优异的电化学性能归因于中等Ni掺杂引起的电子电导率和反应动力学的改善。
更新日期:2020-10-21
中文翻译:

Ni掺杂作为高性能锂离子电池阴极的FeF 3 ·0.33H 2 O纳米球带隙工程
铁基氟化物是一种插层-转换阴极材料,由于其高容量和廉价的成本,被认为是锂离子电池最有希望的候选阴极材料之一。然而,循环时差的电导率和缓慢的反应动力学阻碍了它们的实际应用。此处,Ni掺杂的FeF 3 ·0.33H 2为了克服上述缺陷,已经提出并通过简单的溶剂热法制备了O。通过第一性原理计算和综合的理化特性研究了镍掺杂对物理和电化学性能的影响。结果表明,Ni适当取代Fe位点可导致F空位而不改变本征晶体结构,但不仅减小了带隙,提高了本征电导率,而且拓宽了离子通道,从而促进了离子的快速迁移。和电子获得优异的电化学性能。与裸FeF 3 ·0.33H 2 O相比,适量(8%)的Ni 2+掺杂具有更高的可逆容量,更长的循环稳定性和更好的速率能力。它具有412 mAh g –1的初始放电容量,在0.25 C(1 C = 200 mA g –1)在1.5–4.5 V的范围内经过100个循环后仍保持264 mAh g –1。此外,在2.0 C时,它仍具有仍可提供248 mAh g –1的高放电容量。优异的电化学性能归因于中等Ni掺杂引起的电子电导率和反应动力学的改善。









































 京公网安备 11010802027423号
京公网安备 11010802027423号