当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Capping Agents in the Synthesis of Salicylate-Capped Zinc Oxide Nanoparticles
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-09-17 , DOI: 10.1021/acsanm.0c01972 Takat B. Rawal 1, 2 , Micholas D. Smith 1, 2 , Ali Ozcan 3, 4, 5 , Jeremy C. Smith 1, 2 , Laurene Tetard 3, 6 , Swadeshmukul Santra 3, 4, 7 , Loukas Petridis 1, 2
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-09-17 , DOI: 10.1021/acsanm.0c01972 Takat B. Rawal 1, 2 , Micholas D. Smith 1, 2 , Ali Ozcan 3, 4, 5 , Jeremy C. Smith 1, 2 , Laurene Tetard 3, 6 , Swadeshmukul Santra 3, 4, 7 , Loukas Petridis 1, 2
Affiliation
|
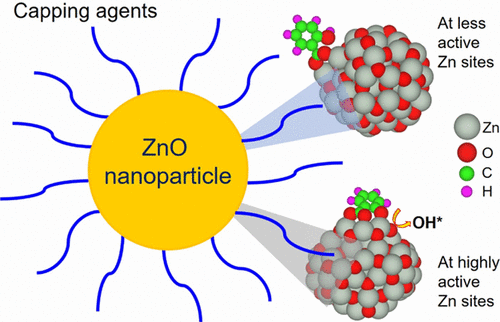
Capping agents are often used for controlling the size, aggregation, and properties of nanoparticles. To guide the design of improved nanomaterials for targeted performance, one can use mechanistic insights into the interactions between capping agents and nanoparticles. Here, we employ density functional theory (DFT), reactive force-field molecular dynamics (ReaxFF MD) simulations, and optical spectroscopy to study the interactions between salicylate, as a model capping agent, and zinc oxide (ZnO) nanoparticles. We find that salicylate strongly interacts with the nanoparticle via the formation of O–Zn bonds in a distorted six-membered coordination ring structure. We describe the mechanisms of capping of ZnO nanoparticles by salicylate via three different binding modes. Simulations indicate that salicylate undergoes dissociative adsorption at the highly active surface Zn sites via a hydrogen-transfer process, thereby forming a tridentate configuration. The water-mediated interaction also facilitates the dissociative adsorption, leading to two salicylate O atoms coordinating with a surface Zn atom, while the other salicylate O atom bonds with another surface Zn atom. For molecular adsorption, binding free energies indicate that salicylate binds more strongly to ZnO, often in a bidentate configuration, than water does. The formation of the salicylate–ZnO complex is substantiated by UV–visible and Fourier transform infrared spectra. We find that the C═O stretching mode of salicylate becomes softened when it interacts with the nanoparticle, suggesting chemisorption of salicylate on ZnO. Although DFT predicts strong interaction between salicylate and ZnO, ReaxFF MD simulation indicates the moderate interaction between these two components in aqueous solution. Water molecules in close contact with the nanoparticle surface undergo dissociation, thus resulting in a surface hydroxyl, a reactive oxygen species that may influence the nanoparticle’s catalytic properties. The atomic-level information provided here can guide the selection process of salicylate as an appropriate agent for ZnO nanoparticle synthesis when strong interactions of particles with capping agents are required.
中文翻译:

封端剂在水杨酸酯封端的氧化锌纳米粒子合成中的作用
封端剂通常用于控制纳米粒子的尺寸,聚集和性质。为了指导针对目标性能的改进纳米材料的设计,人们可以利用机械学的观点来研究封端剂与纳米颗粒之间的相互作用。在这里,我们采用密度泛函理论(DFT),反作用力场分子动力学(ReaxFF MD)模拟和光谱学来研究水杨酸盐(作为模型封端剂)与氧化锌(ZnO)纳米粒子之间的相互作用。我们发现,水杨酸酯通过扭曲的六元配位环结构中的O-Zn键形成,与纳米粒子强烈相互作用。我们描述水杨酸封盖ZnO纳米颗粒的机制通过三种不同的绑定模式。模拟表明,水杨酸酯通过氢转移过程在高活性表面Zn位置经历解离吸附,从而形成三齿构型。水介导的相互作用还促进了解离吸附,导致两个水杨酸酯O原子与一个表面Zn原子配位,而另一个水杨酸酯O原子与另一个表面Zn原子键合。对于分子吸附,结合自由能表明水杨酸酯与水相比,通常以双齿结构更牢固地与ZnO结合。水杨酸盐-ZnO复合物的形成通过紫外可见光和傅立叶变换红外光谱得以证实。我们发现水杨酸酯的C═O拉伸模式在与纳米粒子相互作用时变得软化,表明水杨酸酯在ZnO上的化学吸附。尽管DFT预测了水杨酸盐和ZnO之间有很强的相互作用,但是ReaxFF MD模拟表明这两种组分在水溶液中存在适度的相互作用。与纳米颗粒表面紧密接触的水分子发生解离,从而产生表面羟基,这是一种活性氧,可能会影响纳米颗粒的催化性能。当需要颗粒与封端剂的强相互作用时,此处提供的原子级信息可指导水杨酸盐作为ZnO纳米颗粒合成的合适试剂的选择过程。因此会产生表面羟基,这是一种可能影响纳米颗粒催化性能的活性氧。当需要颗粒与封端剂的强相互作用时,此处提供的原子级信息可指导水杨酸盐作为ZnO纳米颗粒合成的合适试剂的选择过程。因此会产生表面羟基,这是一种可能影响纳米颗粒催化性能的活性氧。当需要颗粒与封端剂的强相互作用时,此处提供的原子级信息可指导水杨酸盐作为ZnO纳米颗粒合成的合适试剂的选择过程。
更新日期:2020-10-25
中文翻译:

封端剂在水杨酸酯封端的氧化锌纳米粒子合成中的作用
封端剂通常用于控制纳米粒子的尺寸,聚集和性质。为了指导针对目标性能的改进纳米材料的设计,人们可以利用机械学的观点来研究封端剂与纳米颗粒之间的相互作用。在这里,我们采用密度泛函理论(DFT),反作用力场分子动力学(ReaxFF MD)模拟和光谱学来研究水杨酸盐(作为模型封端剂)与氧化锌(ZnO)纳米粒子之间的相互作用。我们发现,水杨酸酯通过扭曲的六元配位环结构中的O-Zn键形成,与纳米粒子强烈相互作用。我们描述水杨酸封盖ZnO纳米颗粒的机制通过三种不同的绑定模式。模拟表明,水杨酸酯通过氢转移过程在高活性表面Zn位置经历解离吸附,从而形成三齿构型。水介导的相互作用还促进了解离吸附,导致两个水杨酸酯O原子与一个表面Zn原子配位,而另一个水杨酸酯O原子与另一个表面Zn原子键合。对于分子吸附,结合自由能表明水杨酸酯与水相比,通常以双齿结构更牢固地与ZnO结合。水杨酸盐-ZnO复合物的形成通过紫外可见光和傅立叶变换红外光谱得以证实。我们发现水杨酸酯的C═O拉伸模式在与纳米粒子相互作用时变得软化,表明水杨酸酯在ZnO上的化学吸附。尽管DFT预测了水杨酸盐和ZnO之间有很强的相互作用,但是ReaxFF MD模拟表明这两种组分在水溶液中存在适度的相互作用。与纳米颗粒表面紧密接触的水分子发生解离,从而产生表面羟基,这是一种活性氧,可能会影响纳米颗粒的催化性能。当需要颗粒与封端剂的强相互作用时,此处提供的原子级信息可指导水杨酸盐作为ZnO纳米颗粒合成的合适试剂的选择过程。因此会产生表面羟基,这是一种可能影响纳米颗粒催化性能的活性氧。当需要颗粒与封端剂的强相互作用时,此处提供的原子级信息可指导水杨酸盐作为ZnO纳米颗粒合成的合适试剂的选择过程。因此会产生表面羟基,这是一种可能影响纳米颗粒催化性能的活性氧。当需要颗粒与封端剂的强相互作用时,此处提供的原子级信息可指导水杨酸盐作为ZnO纳米颗粒合成的合适试剂的选择过程。











































 京公网安备 11010802027423号
京公网安备 11010802027423号