当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coupling without Coupling Reactions: En Route to Developing Phenols as Sustainable Coupling Partners via Dearomatization-Rearomatization Processes.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.accounts.0c00479 Zihang Qiu 1 , Huiying Zeng 2 , Chao-Jun Li 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.accounts.0c00479 Zihang Qiu 1 , Huiying Zeng 2 , Chao-Jun Li 1
Affiliation
|
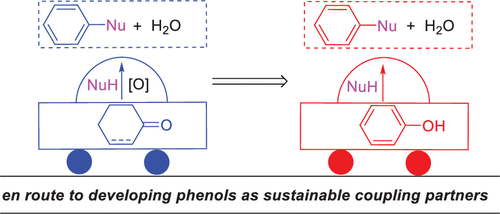
Transition-metal-catalyzed cross-coupling reactions represent one of the most straightforward and efficient protocols to assemble two different molecular motifs for the construction of carbon–carbon or carbon–heteroatom bonds. Because of their importance and wide applications in pharmaceuticals, agrochemicals, materials, etc., cross-coupling reactions have been well recognized in the 2010 Nobel Prize in chemistry. However, in the classical transition-metal-catalyzed cross-coupling reactions (e.g., the Suzuki–Miyaura, the Buchwald–Hartwig, and the Ullmann cross-coupling reactions), organohalides, which mainly stem from the nonrenewable fossil resources, are often utilized as coupling partners with halide wastes being generated after the reactions. To make cross-coupling reactions more sustainable, we initiated a general research program by employing phenols and cyclohexa(e)nones (the reduced forms of phenols) as pivotal feedstocks (coupling partners), instead of the commonly used fossil-derived organohalides, for cross-coupling reactions to build C–O, C–N, and C–C bonds. Phenols (cyclohexa(e)nones) are widely available and can be obtained from lignin biomass, highlighting their renewable and sustainable features. Moreover, water is expected to be the only stoichiometric byproduct, thus avoiding halide wastes.
中文翻译:

没有偶联反应的偶联:在通过脱芳香化-再芳香化过程发展成为可持续的偶联伙伴的苯酚的过程中。
过渡金属催化的交叉偶联反应是组装两个不同的分子基序以构建碳-碳或碳-杂原子键的最直接有效的方法之一。由于它们的重要性和在制药,农用化学品,材料等领域的广泛应用,交叉偶联反应已获得2010年诺贝尔化学奖。但是,在经典的过渡金属催化的交叉偶联反应中(例如,Suzuki–Miyaura,Buchwald–Hartwig和Ullmann交叉偶联反应),通常使用主要来源于不可再生化石资源的有机卤化物作为偶联伙伴,反应后会产生卤化物废物。为了使交叉耦合反应更加可持续,我们通过使用苯酚和环己酮(还原形式的苯酚)作为关键原料(偶联伙伴)而不是常用的化石来源的有机卤化物进行交叉偶联反应来构建C–O,从而启动了一项总体研究计划,C–N和C–C键。苯酚(环己酮)是广泛可用的,可以从木质素生物质中获得,突出了其可再生和可持续的特征。而且,预计水是唯一的化学计量副产物,从而避免了卤化物的浪费。突出其可再生和可持续的特征。此外,预计水是唯一的化学计量副产物,从而避免了卤化物的浪费。突出其可再生和可持续的特征。而且,预计水是唯一的化学计量副产物,从而避免了卤化物的浪费。
更新日期:2020-10-21
中文翻译:

没有偶联反应的偶联:在通过脱芳香化-再芳香化过程发展成为可持续的偶联伙伴的苯酚的过程中。
过渡金属催化的交叉偶联反应是组装两个不同的分子基序以构建碳-碳或碳-杂原子键的最直接有效的方法之一。由于它们的重要性和在制药,农用化学品,材料等领域的广泛应用,交叉偶联反应已获得2010年诺贝尔化学奖。但是,在经典的过渡金属催化的交叉偶联反应中(例如,Suzuki–Miyaura,Buchwald–Hartwig和Ullmann交叉偶联反应),通常使用主要来源于不可再生化石资源的有机卤化物作为偶联伙伴,反应后会产生卤化物废物。为了使交叉耦合反应更加可持续,我们通过使用苯酚和环己酮(还原形式的苯酚)作为关键原料(偶联伙伴)而不是常用的化石来源的有机卤化物进行交叉偶联反应来构建C–O,从而启动了一项总体研究计划,C–N和C–C键。苯酚(环己酮)是广泛可用的,可以从木质素生物质中获得,突出了其可再生和可持续的特征。而且,预计水是唯一的化学计量副产物,从而避免了卤化物的浪费。突出其可再生和可持续的特征。此外,预计水是唯一的化学计量副产物,从而避免了卤化物的浪费。突出其可再生和可持续的特征。而且,预计水是唯一的化学计量副产物,从而避免了卤化物的浪费。











































 京公网安备 11010802027423号
京公网安备 11010802027423号