当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfono-γ-AApeptides as Helical Mimetics: Crystal Structures and Applications.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.accounts.0c00482 Peng Sang 1 , Yan Shi 1 , Bo Huang 1 , Songyi Xue 1 , Timothy Odom 1 , Jianfeng Cai 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-09-17 , DOI: 10.1021/acs.accounts.0c00482 Peng Sang 1 , Yan Shi 1 , Bo Huang 1 , Songyi Xue 1 , Timothy Odom 1 , Jianfeng Cai 1
Affiliation
|
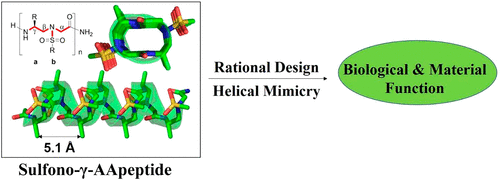
Foldamers have defined and predictable structures, improved resistance to proteolytic degradation, enhanced chemical diversity, and are versatile in their mimicry of biological molecules, making them promising candidates in biomedical and material applications. However, as natural macromolecules exhibit endless folding structures and functions, the exploration of the applications of foldamers remains crucial. As such, it is imperative to continue to discover unnatural foldameric architectures with new frameworks and molecular scaffolds. To this end, we recently developed a new class of peptidomimetics termed ″γ-AApeptides”, oligomers of γ-substituted-N-acylated-N-aminoethyl amino acids, which are inspired by the chiral peptide nucleic acid backbone. To date γ-AApeptides have been shown to be resistant to proteolytic degradation and possess limitless potential to introduce chemically diverse functional groups, demonstrating promise in biomedical and material sciences. However, the structures of γ-AApeptides were initially unknown, rendering their rational design for the mimicry of a protein helical domain impossible in the beginning, which limited their potential development. To our delight, in the past few years, we have obtained a series of crystal structures of helical sulfono-γ-AApeptides, a subclass of γ-AApeptides. The single-crystal X-ray crystallography indicates that sulfono-γ-AApeptides fold into unprecedented and well-defined helices with unique helical parameters. On the basis of the well-established size, shape, and folding conformation, the design of sulfono-γ-AApeptide-based foldamers opens a new avenue for the development of alternative unnatural peptidomimetics for their potential applications in chemistry, biology, medicine, materials science, and so on.
中文翻译:

作为螺旋模拟物的磺基-γ-AA 肽:晶体结构和应用。
折叠分子具有定义和可预测的结构,提高了对蛋白水解降解的抵抗力,增强了化学多样性,并且在模拟生物分子方面具有多种用途,使其在生物医学和材料应用中成为有希望的候选者。然而,由于天然大分子表现出无穷无尽的折叠结构和功能,探索折叠体的应用仍然至关重要。因此,必须继续发现具有新框架和分子支架的非自然折叠结构。为此,我们最近开发了一类新的肽模拟物,称为“γ-A肽”,γ-取代-N-酰化-N的寡聚体-氨基乙基氨基酸,其灵感来自手性肽核酸骨架。迄今为止,γ-AA 肽已被证明对蛋白水解降解具有抗性,并具有引入化学多样性官能团的无限潜力,在生物医学和材料科学中显示出前景。然而,γ-AA肽的结构最初是未知的,使得它们在开始时无法合理设计模拟蛋白质螺旋结构域,这限制了它们的潜在发展。令我们高兴的是,在过去的几年里,我们已经获得了一系列螺旋磺基-γ-AA肽的晶体结构,它是γ-AA肽的一个亚类。单晶 X 射线晶体学表明磺基-γ-AA 肽折叠成具有独特螺旋参数的前所未有且定义明确的螺旋。
更新日期:2020-10-21
中文翻译:

作为螺旋模拟物的磺基-γ-AA 肽:晶体结构和应用。
折叠分子具有定义和可预测的结构,提高了对蛋白水解降解的抵抗力,增强了化学多样性,并且在模拟生物分子方面具有多种用途,使其在生物医学和材料应用中成为有希望的候选者。然而,由于天然大分子表现出无穷无尽的折叠结构和功能,探索折叠体的应用仍然至关重要。因此,必须继续发现具有新框架和分子支架的非自然折叠结构。为此,我们最近开发了一类新的肽模拟物,称为“γ-A肽”,γ-取代-N-酰化-N的寡聚体-氨基乙基氨基酸,其灵感来自手性肽核酸骨架。迄今为止,γ-AA 肽已被证明对蛋白水解降解具有抗性,并具有引入化学多样性官能团的无限潜力,在生物医学和材料科学中显示出前景。然而,γ-AA肽的结构最初是未知的,使得它们在开始时无法合理设计模拟蛋白质螺旋结构域,这限制了它们的潜在发展。令我们高兴的是,在过去的几年里,我们已经获得了一系列螺旋磺基-γ-AA肽的晶体结构,它是γ-AA肽的一个亚类。单晶 X 射线晶体学表明磺基-γ-AA 肽折叠成具有独特螺旋参数的前所未有且定义明确的螺旋。











































 京公网安备 11010802027423号
京公网安备 11010802027423号