当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One‐pot synthesis of novel benzimidazoles with a naphthalene moiety as antimicrobial agents and molecular docking studies
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-09-17 , DOI: 10.1002/jccs.202000125 Ronak Haj Ersan 1 , Ahmet Yuksel 1 , Tugba Ertan‐Bolelli 2 , Aylin Dogen 3 , Serdar Burmaoglu 4 , Oztekin Algul 1
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2020-09-17 , DOI: 10.1002/jccs.202000125 Ronak Haj Ersan 1 , Ahmet Yuksel 1 , Tugba Ertan‐Bolelli 2 , Aylin Dogen 3 , Serdar Burmaoglu 4 , Oztekin Algul 1
Affiliation
|
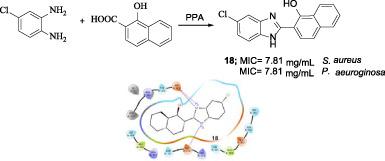
In an attempt to design a greener approach for the synthesis of a potent class of antimicrobials, 1,2‐phenylenediamine derivatives were reacted with various 1/2‐carboxylic acid‐substituted naphthalene derivatives to generate a series of naphthyl‐substituted benzimidazole derivatives (11–19) using polyphosphoric acid as catalyst under microwave irradiation and conventional synthesis method. This is an eco‐friendly and swift reaction method for a synthetic approach to diverse benzimidazoles. Structures of the synthesized compounds were established on the basis of spectral data and they were screened for their antimicrobial activity. Compound 18 showed maximum potency against all Gram‐positive and Gram‐negative bacterial strains with a minimum inhibitory concentration (MIC) value in the range of 7.81–62.50 μg/ml. Only compound 17 was found to be the most active against all fungal strains with a MIC value of 15.62 μg/ml. In this study, we performed molecular docking experiments to understand the interactions between compounds 17 and 18 and E. coli topoisomerase I, and we compared the results obtained with that of 2‐(3,4‐dimethoxyphenyl)‐5‐[5‐(4‐methylpiperazin−1‐yl)‐1H‐benzimidazol‐2‐yl]‐1H‐benzimidazole (DMA). Compounds 17 and 18 demonstrated strong interactions with important active site residues, similar to DMA. As a result, the compounds obtained from this study can be used in designing new potent inhibitors of E. coli topoisomerase I.
中文翻译:

一锅合成具有萘部分的新型苯并咪唑作为抗菌剂和分子对接研究
为了设计一种更绿色的方法来合成强大的抗菌剂,将1,2-苯二胺衍生物与各种1 / 2-羧酸取代的萘衍生物反应,生成了一系列萘基取代的苯并咪唑衍生物(11 – 19)在微波辐射和常规合成方法下,使用多磷酸作为催化剂。这是一种环保,快速的反应方法,适用于多种苯并咪唑的合成方法。根据光谱数据确定合成化合物的结构,并筛选其抗菌活性。化合物18在所有革兰氏阳性和革兰氏阴性细菌菌株中显示最大效价,最小抑菌浓度(MIC)值在7.81–62.50μg/ ml范围内。发现仅化合物17对所有真菌菌株最具活性,其MIC值为15.62μg/ ml。在这项研究中,我们进行了分子对接实验,以了解化合物17和18与大肠杆菌拓扑异构酶I之间的相互作用,并将我们获得的结果与2-(3,4-二甲氧基苯基)-5- [5-( 4-甲基哌嗪-1-基)-1H-苯并咪唑-2-基] -1H-苯并咪唑(DMA)。化合物17和18与重要的活性位点残基具有很强的相互作用,类似于DMA。结果,从这项研究中获得的化合物可用于设计大肠杆菌拓扑异构酶I的新型有效抑制剂。
更新日期:2020-09-17
中文翻译:

一锅合成具有萘部分的新型苯并咪唑作为抗菌剂和分子对接研究
为了设计一种更绿色的方法来合成强大的抗菌剂,将1,2-苯二胺衍生物与各种1 / 2-羧酸取代的萘衍生物反应,生成了一系列萘基取代的苯并咪唑衍生物(11 – 19)在微波辐射和常规合成方法下,使用多磷酸作为催化剂。这是一种环保,快速的反应方法,适用于多种苯并咪唑的合成方法。根据光谱数据确定合成化合物的结构,并筛选其抗菌活性。化合物18在所有革兰氏阳性和革兰氏阴性细菌菌株中显示最大效价,最小抑菌浓度(MIC)值在7.81–62.50μg/ ml范围内。发现仅化合物17对所有真菌菌株最具活性,其MIC值为15.62μg/ ml。在这项研究中,我们进行了分子对接实验,以了解化合物17和18与大肠杆菌拓扑异构酶I之间的相互作用,并将我们获得的结果与2-(3,4-二甲氧基苯基)-5- [5-( 4-甲基哌嗪-1-基)-1H-苯并咪唑-2-基] -1H-苯并咪唑(DMA)。化合物17和18与重要的活性位点残基具有很强的相互作用,类似于DMA。结果,从这项研究中获得的化合物可用于设计大肠杆菌拓扑异构酶I的新型有效抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号