当前位置:
X-MOL 学术
›
J. Appl. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalized chitosan with butylammonium ionic liquids for removal of Cr(VI) from aqueous solution
Journal of Applied Polymer Science ( IF 2.7 ) Pub Date : 2020-09-17 , DOI: 10.1002/app.49912 Kevy Pontes Eliodório 1 , Gilberto José Pereira 2 , Andreia Morandim‐Giannetti 1
Journal of Applied Polymer Science ( IF 2.7 ) Pub Date : 2020-09-17 , DOI: 10.1002/app.49912 Kevy Pontes Eliodório 1 , Gilberto José Pereira 2 , Andreia Morandim‐Giannetti 1
Affiliation
|
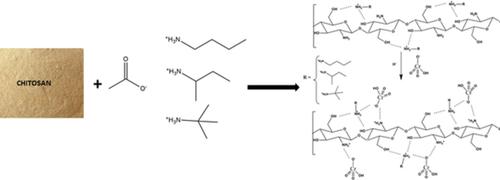
Environmental pollution by heavy metals is currently a problem of great concern for human health. In this context, this study aims to contribute with the synthesis and characterization of chitosan functionalized with three different ionic liquids (n‐butylammonium acetate, sec‐butylammonium acetate, and tert‐butylammonium acetate) followed by its application in hexavalent chromium effluent treatment. The adsorbents synthesized (ChN, ChS, and ChT) were characterized by SEM, EDS, FTIR, BET, RDD, PSD, and XRD techniques. Afterward, the influences of temperature, contact time, and pH on the Cr(VI) adsorption process were evaluated. The solution with pH 3 displayed the highest adsorption capacities (107.31, 104.60, and 107.97 mg.g‐1 for ChN, ChS, and ChT, respectively). The kinetic data were better adjusted to the Weber‐Morris kinetic model with an ideal time of 2 h. Furthermore, the influence of temperature was evaluated using the Freundlich and Langmuir isotherms, with maximum capacities of 142.05 (ChN), 131.58 (ChS), and 146.63 mg.g‐1 (ChT). The adsorbent displayed enhanced adsorption properties in comparison with raw chitosan by an intensification of the electrostatic interaction between amino groups and hexavalent chromium. Finally, the reusability was investigated, and significant results were observed (84.33 ± 4.87%) in the adsorption process after 4 cycles.
中文翻译:

含丁基铵离子液体的功能化壳聚糖,用于从水溶液中去除Cr(VI)
目前,重金属对环境的污染已成为人类健康的重大问题。在此背景下,本研究旨在为被三种不同离子液体(乙酸正丁铵,乙酸仲丁铵和乙酸叔丁铵)官能化的壳聚糖的合成和表征做出贡献,然后将其应用于六价铬废水处理中。合成的吸附剂(ChN,ChS和ChT)通过SEM,EDS,FTIR,BET,RDD,PSD和XRD技术进行了表征。然后,评估了温度,接触时间和pH对Cr(VI)吸附过程的影响。pH为3的溶液显示出最高的吸附容量(107.31、104.60和107.97 mg.g -1分别用于ChN,ChS和ChT)。动力学数据可以在2 h的理想时间内更好地适应Weber-Morris动力学模型。此外,使用Freundlich和Langmuir等温线评估了温度的影响,最大容量为142.05(ChN),131.58(ChS)和146.63 mg.g -1(ChT)。与未加工的壳聚糖相比,该吸附剂通过增强氨基和六价铬之间的静电相互作用而显示出增强的吸附性能。最后,对可重复使用性进行了研究,并在4个循环后的吸附过程中观察到了显着的结果(84.33±4.87%)。
更新日期:2020-11-25
中文翻译:

含丁基铵离子液体的功能化壳聚糖,用于从水溶液中去除Cr(VI)
目前,重金属对环境的污染已成为人类健康的重大问题。在此背景下,本研究旨在为被三种不同离子液体(乙酸正丁铵,乙酸仲丁铵和乙酸叔丁铵)官能化的壳聚糖的合成和表征做出贡献,然后将其应用于六价铬废水处理中。合成的吸附剂(ChN,ChS和ChT)通过SEM,EDS,FTIR,BET,RDD,PSD和XRD技术进行了表征。然后,评估了温度,接触时间和pH对Cr(VI)吸附过程的影响。pH为3的溶液显示出最高的吸附容量(107.31、104.60和107.97 mg.g -1分别用于ChN,ChS和ChT)。动力学数据可以在2 h的理想时间内更好地适应Weber-Morris动力学模型。此外,使用Freundlich和Langmuir等温线评估了温度的影响,最大容量为142.05(ChN),131.58(ChS)和146.63 mg.g -1(ChT)。与未加工的壳聚糖相比,该吸附剂通过增强氨基和六价铬之间的静电相互作用而显示出增强的吸附性能。最后,对可重复使用性进行了研究,并在4个循环后的吸附过程中观察到了显着的结果(84.33±4.87%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号