当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Separation of Intrinsically Disordered Protein Polymers Mechanically Stiffens Fibrin Clots
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-09-16 , DOI: 10.1002/adfm.202005245 Ivan Urosev 1, 2 , Joanan Lopez Morales 1, 2 , Michael A. Nash 1, 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-09-16 , DOI: 10.1002/adfm.202005245 Ivan Urosev 1, 2 , Joanan Lopez Morales 1, 2 , Michael A. Nash 1, 2
Affiliation
|
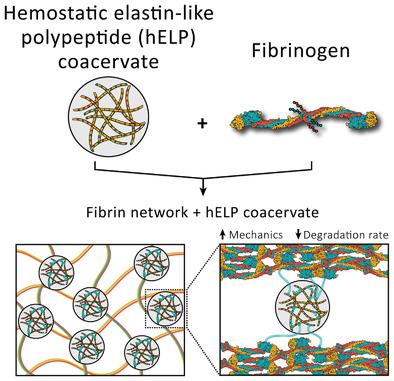
Fibrin (Fb) networks self‐assemble through the coagulation cascade and serve as the structural foundation of blood clots. Following severe trauma or drug therapy, reduced integrity of Fb networks can lead to formation of clots with inadequate mechanical properties. A key feature of therapeutic interventions for hemostasis is therefore the ability to restore mechanical strength to clots formed under coagulopathic conditions. Here, an intrinsically disordered protein based on an elastin‐like polypeptide (ELP) sequence is described, which specifically binds Fb and modulates its mechanical properties. Hemostatic ELPs (hELPs) are designed containing N‐ and C‐terminal peptide tags that are selectivity recognized by human transglutaminase factor XIIIa and covalently linked into fibrin networks via the natural coagulation cascade. Phase separation of hELPs above their lower critical solution temperature leads to stiffening and rescue of clot biophysical properties under simulated conditions of dilutive coagulopathy. In addition to phase‐dependent stiffening, the resulting hELP‐Fb networks exhibit resistance to plasmin degradation, reduced pore sizes, and accelerated gelation rate following initiation of clotting. These results demonstrate the ability of protein‐based phase separation to modulate the physical and biochemical properties of blood clots and suggest protein phase separation as a new mechanism for achieving hemostasis in clinical settings.
中文翻译:

固有紊乱的蛋白质聚合物的相分离机械增强纤维蛋白凝块。
纤维蛋白(Fb)网络通过凝血级联反应自组装,并作为血凝块的结构基础。经过严重的创伤或药物治疗后,Fb网络的完整性降低会导致形成凝块,而凝块的机械性能不足。因此,止血治疗干预措施的关键特征是能够恢复机械强度至凝血病条件下形成的血凝块的能力。此处描述了一种基于弹性蛋白样多肽(ELP)序列的内在无序蛋白,其特异性结合Fb并调节其机械性能。止血ELP(hELP)设计为包含N和C末端肽标签,这些标签被人类转谷氨酰胺酶因子XIIIa识别并通过自然凝血级联共价连接到血纤蛋白网络中。在稀释性凝血病的模拟条件下,高于最低临界溶解温度的hELP的相分离会导致凝块生物物理特性的变硬和恢复。除了依赖于相位的硬化外,生成的hELP-Fb网络还显示出对血纤维蛋白溶酶降解的抵抗力,减小的孔径以及在凝结后加速的胶凝速率。这些结果证明了基于蛋白质的相分离能够调节血凝块的物理和生化特性,并表明蛋白质相分离是在临床环境中实现止血的新机制。产生的hELP-Fb网络对血纤维蛋白溶酶降解具有抵抗力,减小了孔径,并在凝结后加速了凝胶化速度。这些结果证明了基于蛋白质的相分离能够调节血凝块的物理和生化特性,并表明蛋白质相分离是在临床环境中实现止血的新机制。产生的hELP-Fb网络对血纤维蛋白溶酶降解具有抵抗力,减小了孔径,并在凝结后加速了凝胶化速度。这些结果证明了基于蛋白质的相分离能够调节血凝块的物理和生化特性,并表明蛋白质相分离是在临床环境中实现止血的新机制。
更新日期:2020-09-16
中文翻译:

固有紊乱的蛋白质聚合物的相分离机械增强纤维蛋白凝块。
纤维蛋白(Fb)网络通过凝血级联反应自组装,并作为血凝块的结构基础。经过严重的创伤或药物治疗后,Fb网络的完整性降低会导致形成凝块,而凝块的机械性能不足。因此,止血治疗干预措施的关键特征是能够恢复机械强度至凝血病条件下形成的血凝块的能力。此处描述了一种基于弹性蛋白样多肽(ELP)序列的内在无序蛋白,其特异性结合Fb并调节其机械性能。止血ELP(hELP)设计为包含N和C末端肽标签,这些标签被人类转谷氨酰胺酶因子XIIIa识别并通过自然凝血级联共价连接到血纤蛋白网络中。在稀释性凝血病的模拟条件下,高于最低临界溶解温度的hELP的相分离会导致凝块生物物理特性的变硬和恢复。除了依赖于相位的硬化外,生成的hELP-Fb网络还显示出对血纤维蛋白溶酶降解的抵抗力,减小的孔径以及在凝结后加速的胶凝速率。这些结果证明了基于蛋白质的相分离能够调节血凝块的物理和生化特性,并表明蛋白质相分离是在临床环境中实现止血的新机制。产生的hELP-Fb网络对血纤维蛋白溶酶降解具有抵抗力,减小了孔径,并在凝结后加速了凝胶化速度。这些结果证明了基于蛋白质的相分离能够调节血凝块的物理和生化特性,并表明蛋白质相分离是在临床环境中实现止血的新机制。产生的hELP-Fb网络对血纤维蛋白溶酶降解具有抵抗力,减小了孔径,并在凝结后加速了凝胶化速度。这些结果证明了基于蛋白质的相分离能够调节血凝块的物理和生化特性,并表明蛋白质相分离是在临床环境中实现止血的新机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号