Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-09-17 , DOI: 10.1016/j.jhazmat.2020.123988 Zhendong Yang , Joanna Karczewska-Golec , Michal Styczynski , Tomasz Bajda , Lukasz Drewniak
|
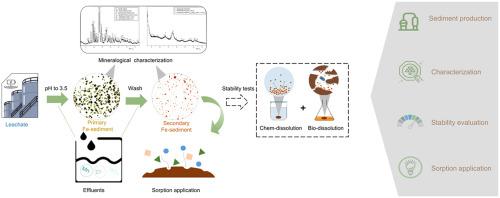
The waste leachate from the hydrometallurgical recycling of spent batteries contains a significant amount of undesirable iron that needs to be precipitated before the recovery of target metals. The produced Fe-sediments are usually disposed of or stored at the treatment site as waste and are often poorly managed. This work estimates the environmental stability and application potential of Fe-sediments produced from highly acidic hydrometallurgical leachate during the recycling of spent alkaline batteries. After pH neutralization of the leachate by Na2CO3, a primary Fe-sediment (PFS), mainly composed of highly unstable metal (i.e., Fe, Zn, and Mn) sulfates, was obtained. The subsequent rinsing of this unstable PFS sediment led to the production of a secondary Fe-sediment (SFS), which was composed of an amorphous-phased ferric iron sulfate hydrate – Fe16O16(SO4)3(OH)10·10H2O. The results of single extraction using chemical reagents and biological dissolution by iron-transforming bacteria confirmed that despite most of the ions in PFS were dissolvable, the processed SFS was environmentally safe. The sorption efficiency of SFS towards Pb(II) and As(V) (up to ~99% and 94%, respectively, with an initial concentration of 100 mg/L) was found to be promising, suggesting the high potential for economical reuse of SFS .
中文翻译:

湿法冶金废液的化学预处理从碱性电池的回收中获得的铁基沉积物的特性
来自废电池的湿法冶金回收的废渗滤液含有大量不希望的铁,在回收目标金属之前需要将其沉淀。产生的铁沉积物通常作为废物处置或存储在处理场所,并且管理不善。这项工作估计了在废旧碱性电池的回收过程中由高酸性湿法冶金浸出液生产的铁沉淀物的环境稳定性和应用潜力。用Na 2 CO 3中和渗滤液的pH后获得了主要由高度不稳定的金属(即Fe,Zn和Mn)硫酸盐组成的初级Fe沉积物(PFS)。随后漂洗这种不稳定的PFS沉积物,产生了次级Fe沉积物(SFS),该沉积物由非晶相硫酸铁水合物– Fe 16 O 16(SO 4)3(OH)10 ·10H组成2O.使用化学试剂进行单次提取以及通过铁转化细菌进行生物溶解的结果证实,尽管PFS中的大多数离子都是可溶的,但经过处理的SFS对环境安全。发现SFS对Pb(II)和As(V)的吸附效率(分别为〜99%和94%,初始浓度为100 mg / L)是有前途的,表明了经济再利用的巨大潜力SFS。










































 京公网安备 11010802027423号
京公网安备 11010802027423号