当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pd-doped C3N monolayer: a promising low-temperature and high-activity single-atom catalyst for CO oxidation
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.apsusc.2020.147881 Hao Cui , Zhongqi Liu , Pengfei Jia
Applied Surface Science ( IF 6.3 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.apsusc.2020.147881 Hao Cui , Zhongqi Liu , Pengfei Jia
|
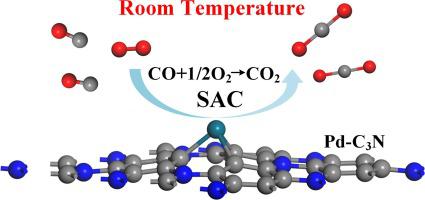
Abstract Using the first-principles theory, we theoretically investigated the CO/O2 adsorption and CO oxidation on the Pd-doped C3N (Pd-C3N) monolayer which we proposed as a low-temperature and high-activity catalyst for CO oxidation. Pd dopant is stably anchored on the N vacancy of C3N monolayer, forming the large binding energy of −4.00 eV without the cluster possibility. Given the larger adsorption energy of Pd-C3N monolayer upon O2 compared to CO molecule, we assume that Eley-Rideal (ER) mechanism is the preferred pathway for CO oxidation. For comparison, we also implemented the Langmuir-Hinshelwood (LH) mechanism to comprehensive understand the CO oxidation processes. Our calculations indicated that the energy barriers for ER and LH in the first step are 0.64 and 0.72 eV, with quite large energy drop of 2.99 and 2.0 eV to release a CO2 molecule, respectively. That means, the CO oxidation using Pd-C3N monolayer is fully-energetically favorable even at room temperature, which could give a novel insight into developing novel single-atom catalyst (SAC) based on C3N monolayer with high-efficiency and low-temperature.
中文翻译:

Pd掺杂的C3N单层:一种用于CO氧化的有前途的低温高活性单原子催化剂
摘要 利用第一性原理理论,我们从理论上研究了 Pd 掺杂的 C3N (Pd-C3N) 单层上的 CO/O2 吸附和 CO 氧化,我们提出作为 CO 氧化的低温高活性催化剂。Pd 掺杂剂稳定地锚定在 C3N 单层的 N 空位上,形成 -4.00 eV 的大结合能而没有簇的可能性。鉴于与 CO 分子相比,Pd-C3N 单层在 O2 上的吸附能更大,我们假设 Eley-Rideal (ER) 机制是 CO 氧化的首选途径。为了进行比较,我们还实施了 Langmuir-Hinshelwood (LH) 机制来全面了解 CO 氧化过程。我们的计算表明,第一步中 ER 和 LH 的能垒分别为 0.64 和 0.72 eV,能量下降非常大,分别为 2.99 和 2。0 eV 分别释放一个 CO2 分子。这意味着,即使在室温下,使用 Pd-C3N 单层的 CO 氧化也是完全能量有利的,这可以为开发基于 C3N 单层的高效低温新型单原子催化剂(SAC)提供新的见解。
更新日期:2021-01-01
中文翻译:

Pd掺杂的C3N单层:一种用于CO氧化的有前途的低温高活性单原子催化剂
摘要 利用第一性原理理论,我们从理论上研究了 Pd 掺杂的 C3N (Pd-C3N) 单层上的 CO/O2 吸附和 CO 氧化,我们提出作为 CO 氧化的低温高活性催化剂。Pd 掺杂剂稳定地锚定在 C3N 单层的 N 空位上,形成 -4.00 eV 的大结合能而没有簇的可能性。鉴于与 CO 分子相比,Pd-C3N 单层在 O2 上的吸附能更大,我们假设 Eley-Rideal (ER) 机制是 CO 氧化的首选途径。为了进行比较,我们还实施了 Langmuir-Hinshelwood (LH) 机制来全面了解 CO 氧化过程。我们的计算表明,第一步中 ER 和 LH 的能垒分别为 0.64 和 0.72 eV,能量下降非常大,分别为 2.99 和 2。0 eV 分别释放一个 CO2 分子。这意味着,即使在室温下,使用 Pd-C3N 单层的 CO 氧化也是完全能量有利的,这可以为开发基于 C3N 单层的高效低温新型单原子催化剂(SAC)提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号