当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Angular ladder-type meta-phenylenes: synthesis and electronic structural analysis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-16 , DOI: 10.1039/d0qo00924e Anitha Boddeda 1 , Mohammad Mosharraf Hossain 1 , M Saeed Mirzaei 2 , Sergey V Lindeman 1 , Saber Mirzaei 3 , Rajendra Rathore 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-16 , DOI: 10.1039/d0qo00924e Anitha Boddeda 1 , Mohammad Mosharraf Hossain 1 , M Saeed Mirzaei 2 , Sergey V Lindeman 1 , Saber Mirzaei 3 , Rajendra Rathore 1
Affiliation
|
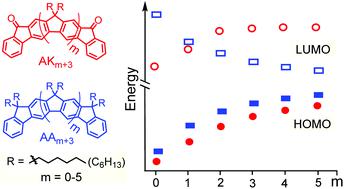
Herein, we report the synthesis of two new series of angular (all-syn) ladder-type meta-[n]phenylenes (LMP, n = 3–8). One series contains keto groups at the termini bridges, denoted angular keto (AKn), the second contains alkyl groups at all bridge sp3 carbons, denoted angular alkyl (AAn). Their electronic and structural properties were delineated by a combination of electrochemistry and spectroscopic (UV-Vis and emission) methods and further supported by DFT calculations. Interestingly, experimental and DFT data show that changing the bridging group at the termini from alkyl (AAn) to keto (AKn) gives an increase in the first reduction potentials and LUMO energies, as the π-system is extended. Also, the charge (de)localization behavior is different for these two species; while the AAn compounds stablize charge with Robin-Day class III, the AKn compounds show a clear switch from class III to class II. In comparison with the linear analogues (LKn and LAn), DFT results reveal a shape independency of the charge (de)localization mechanism in acceptor–π-acceptor series (AKn/LKn).
中文翻译:

角梯型间亚苯基:合成和电子结构分析
在此,我们报告了两个新系列的有角(全同式)梯型间-[ n ]亚苯基(LMP, n = 3-8)的合成。一个系列在末端桥上含有酮基,表示为角酮(AK n ),第二个系列在所有桥sp 3 个碳上含有烷基,表示为角烷基(AA n )。它们的电子和结构特性通过电化学和光谱(紫外-可见光和发射)方法的结合来描述,并得到 DFT 计算的进一步支持。有趣的是,实验和 DFT 数据表明,随着 π 系统的扩展,将末端的桥连基团从烷基 (AA n ) 更改为酮基 (AK n ) 会增加第一还原电位和 LUMO 能量。此外,这两种物质的电荷(去)局域行为也不同。 AA n化合物可稳定 Robin-Day III 类电荷,而 AK n化合物则显示出从 III 类到 II 类的明显转变。与线性类似物(LK n和 LA n )相比,DFT 结果揭示了受体-π-受体系列(AK n /LK n )中电荷(去)局域机制的形状独立性。
更新日期:2020-10-13
中文翻译:

角梯型间亚苯基:合成和电子结构分析
在此,我们报告了两个新系列的有角(全同式)梯型间-[ n ]亚苯基(LMP, n = 3-8)的合成。一个系列在末端桥上含有酮基,表示为角酮(AK n ),第二个系列在所有桥sp 3 个碳上含有烷基,表示为角烷基(AA n )。它们的电子和结构特性通过电化学和光谱(紫外-可见光和发射)方法的结合来描述,并得到 DFT 计算的进一步支持。有趣的是,实验和 DFT 数据表明,随着 π 系统的扩展,将末端的桥连基团从烷基 (AA n ) 更改为酮基 (AK n ) 会增加第一还原电位和 LUMO 能量。此外,这两种物质的电荷(去)局域行为也不同。 AA n化合物可稳定 Robin-Day III 类电荷,而 AK n化合物则显示出从 III 类到 II 类的明显转变。与线性类似物(LK n和 LA n )相比,DFT 结果揭示了受体-π-受体系列(AK n /LK n )中电荷(去)局域机制的形状独立性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号