当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-catalyzed cycloisomerization of ester-tethered 1,6-diynes with cyclopropanol moiety leading to tetralone/exocyclic diene hybrid molecules.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-09-16 , DOI: 10.1039/d0cc05429a Takeshi Yasui 1 , Tomohiro Kikuchi 1 , Yoshihiko Yamamoto 1
Chemical Communications ( IF 4.3 ) Pub Date : 2020-09-16 , DOI: 10.1039/d0cc05429a Takeshi Yasui 1 , Tomohiro Kikuchi 1 , Yoshihiko Yamamoto 1
Affiliation
|
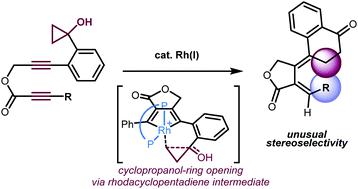
The rhodium-catalyzed cycloisomerization of ester-tethered 1,6-diynes bearing a cyclopropanol moiety produced tetralone/exocyclic diene hybrid molecules with thermodynamically unfavorable alkene geometry. The results of control experiments and density functional theory calculations suggest that the ester tether plays an important role in the efficiency of E/Z isomerization processes.
中文翻译:

铑催化的酯键合的1,6-二炔与环丙醇部分的环异构化反应,导致四氢萘酮/外环二烯杂化分子。
带有环丙醇部分的酯基连接的1,6-二炔的铑催化的环异构化反应产生了四烯酮/环外二烯杂化分子,其具有热力学上不利的烯烃几何形状。控制实验和密度泛函理论计算的结果表明,酯系链在E / Z异构化过程的效率中起着重要作用。
更新日期:2020-09-24
中文翻译:

铑催化的酯键合的1,6-二炔与环丙醇部分的环异构化反应,导致四氢萘酮/外环二烯杂化分子。
带有环丙醇部分的酯基连接的1,6-二炔的铑催化的环异构化反应产生了四烯酮/环外二烯杂化分子,其具有热力学上不利的烯烃几何形状。控制实验和密度泛函理论计算的结果表明,酯系链在E / Z异构化过程的效率中起着重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号