当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Reactivity of Remarkably Stable and Nucleophilic Hydroxide-Bridged Dimetallic Nickel NHC Complexes
Organometallics ( IF 2.5 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.organomet.0c00495 Simone Bertini 1 , Martin Albrecht 1
Organometallics ( IF 2.5 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.organomet.0c00495 Simone Bertini 1 , Martin Albrecht 1
Affiliation
|
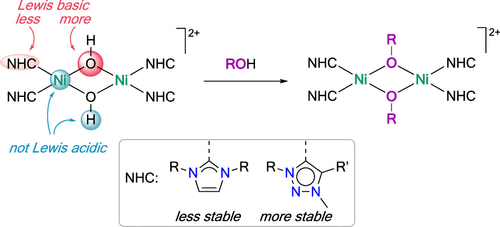
The novel class of dicationic homodimetallic nickel(II) NHC complexes [(NHC)2Ni(μ-OH)2Ni(NHC)2]2+ was synthesized starting from nickel acetate as the metal precursor. Symmetrically substituted N-alkyl (methyl, isopropyl, and isobutyl) imidazolylidene (imi) as well as N1-methyl-, N1-phenyl-, N1-mesityl-, and N1-butyl-substituted triazolylidene (trz) ligands were coordinated to the metal center through NaH-mediated metalation. Reaction of these bimetallic complexes with CH3+ as an electrophile (MeOTf) induced alkylation of the bridging hydroxide ligands and afforded the new alkoxy-bridged complexes [(NHC)2Ni(μ-OMe)2Ni(NHC)2]2+. In contrast, reactions of [(imi)2Ni(μ-OH)2Ni(imi)2]2+ with H+ as electrophile (mild acids with pKa > 6) led to cleavage of the dimeric structure and formation of mononuclear complexes [Ni(X)2(imi)2]. Conversely, no reaction occurred for the triazolylidene analogues [(trz)2Ni(μ-OH)2Ni(trz)2]2+, indicating different robustness of the dimetallic core to acidic media. Only exposure to stronger acids (pKa < 5) induced dimer cleavage for the trz complexes and gave either the corresponding triazolium salt or, in the presence of a coordinating anion, the monomeric species [Ni(X)2(trz)2] with X = I, OAc or (X)2 = CO3. All complexes were inert toward Lewis and Brønsted bases such as NEt3 and NaOMe. These results reveal a remarkable robustness of the acidic Ni centers and the OH protons toward bases and is in contrast with the distinct reactivity of the Lewis basic oxygen donor sites, which are more reactive than the carbene toward electrophiles.
中文翻译:

明显稳定且亲核的氢氧化桥双金属镍NHC配合物的合成和反应性
以乙酸镍为金属前驱体,合成了新型的双金属双金属镍(II)NHC络合物[(NHC)2 Ni(μ-OH)2 Ni(NHC)2 ] 2+。对称取代的N-烷基(甲基,异丙基和异丁基)咪唑基亚烷基(imi)以及N1-甲基,N1-苯基,N1-间甲苯基和N1-丁基取代的三唑基(trz)配体与金属中心通过NaH介导的金属化。这些双金属配合物与CH 3 +作为亲电子试剂(MeOTf)的反应诱导了桥接的氢氧化物配体的烷基化,并提供了新的烷氧基桥接配合物[(NHC)2 Ni(μ-OMe)2Ni(NHC)2 ] 2+。相反,[(imi)2 Ni(μ-OH)2 Ni(imi)2 ] 2+与作为亲电子体的H +(p K a > 6的弱酸)的反应导致二聚体结构的裂解并形成单核络合物[Ni(X)2(imi)2 ]。相反,对于三唑基类似物[(trz)2 Ni(μ-OH)2 Ni(trz)2 ] 2 +没有反应,表明双金属核对酸性介质的耐受性不同。仅暴露于强酸(p K a<5)诱导trz络合物的二聚体裂解,并给出相应的三唑鎓盐,或在存在配位阴离子的情况下,得到X = I,OAc或(X的单体物种[Ni(X)2(trz)2 ] )2 = CO 3。所有复合物对Lewis和Brønsted碱基(例如NEt 3和NaOMe)呈惰性。这些结果揭示了酸性Ni中心和OH质子对碱的出色鲁棒性,并且与Lewis碱性供氧体的独特反应性相反,该反应性比卡宾对亲电体的反应性强。
更新日期:2020-09-28
中文翻译:

明显稳定且亲核的氢氧化桥双金属镍NHC配合物的合成和反应性
以乙酸镍为金属前驱体,合成了新型的双金属双金属镍(II)NHC络合物[(NHC)2 Ni(μ-OH)2 Ni(NHC)2 ] 2+。对称取代的N-烷基(甲基,异丙基和异丁基)咪唑基亚烷基(imi)以及N1-甲基,N1-苯基,N1-间甲苯基和N1-丁基取代的三唑基(trz)配体与金属中心通过NaH介导的金属化。这些双金属配合物与CH 3 +作为亲电子试剂(MeOTf)的反应诱导了桥接的氢氧化物配体的烷基化,并提供了新的烷氧基桥接配合物[(NHC)2 Ni(μ-OMe)2Ni(NHC)2 ] 2+。相反,[(imi)2 Ni(μ-OH)2 Ni(imi)2 ] 2+与作为亲电子体的H +(p K a > 6的弱酸)的反应导致二聚体结构的裂解并形成单核络合物[Ni(X)2(imi)2 ]。相反,对于三唑基类似物[(trz)2 Ni(μ-OH)2 Ni(trz)2 ] 2 +没有反应,表明双金属核对酸性介质的耐受性不同。仅暴露于强酸(p K a<5)诱导trz络合物的二聚体裂解,并给出相应的三唑鎓盐,或在存在配位阴离子的情况下,得到X = I,OAc或(X的单体物种[Ni(X)2(trz)2 ] )2 = CO 3。所有复合物对Lewis和Brønsted碱基(例如NEt 3和NaOMe)呈惰性。这些结果揭示了酸性Ni中心和OH质子对碱的出色鲁棒性,并且与Lewis碱性供氧体的独特反应性相反,该反应性比卡宾对亲电体的反应性强。











































 京公网安备 11010802027423号
京公网安备 11010802027423号