当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CO2 Capture from Biomass Gasification Producer Gas Using a Novel Calcium and Iron-Based Sorbent through Carbonation–Calcination Looping
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.iecr.0c03025 Forogh Dashtestani 1 , Mohammad Nusheh 2 , Vilailuck Siriwongrungson 3 , Janjira Hongrapipat 4 , Vlatko Materic 2 , Shusheng Pang 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-09-16 , DOI: 10.1021/acs.iecr.0c03025 Forogh Dashtestani 1 , Mohammad Nusheh 2 , Vilailuck Siriwongrungson 3 , Janjira Hongrapipat 4 , Vlatko Materic 2 , Shusheng Pang 1
Affiliation
|
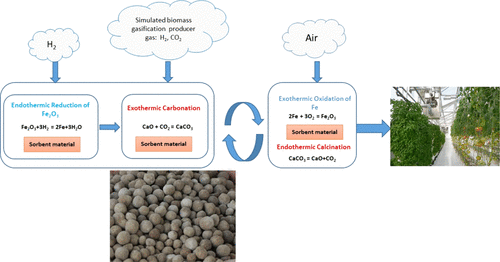
In this study, a novel sorbent material based on CaO and Fe2O3 was investigated for its performance in CO2 capture from simulated biomass gasification producer gas. Experiments were conducted in a fixed bed reactor and each run of the experiments included three major stages of sorbent material reduction, CO2 capture (carbonation), and CO2 release (calcination). The operation temperature in the CO2 capture stage was controlled at 590, 620, 650, and 680 °C, while the temperature for the CO2 release was maintained at 850 °C. The duration of the CO2 capture stage was 3 h and that of the CO2 release stage was 2 h. The effect of cycles of carbonation–calcination looping of the sorbent material was also investigated at the carbonation temperature of 650 °C. The experimental results show that effective CO2 capture by the sorbent material occurred in the initial 20 min during the carbonation. In the calcination stage, the rate of CO2 release reached the peak in 30–40 min from the start of the calcination. The carbonation temperature has a significant effect on CO2 capture and the optimum carbonation temperature was found to be 620 °C, at which CO2 capture efficiency was 94% for the first cycle and 90.4% as average for the first three cycles. It was also found that the CO2 capture efficiency was reduced with cycling. Mechanisms of the CO2 capture and the effect of cycling were also examined.
中文翻译:

使用新型钙和铁基吸收剂通过碳化-煅烧循环从生物质气化生产气中捕获CO 2
在这项研究中,研究了一种基于CaO和Fe 2 O 3的新型吸附剂材料在模拟生物质气化生产气中捕获CO 2中的性能。实验是在固定床反应器中进行的,实验的每轮操作都包括吸附剂还原,CO 2捕集(碳酸化)和CO 2释放(煅烧)三个主要阶段。将CO 2捕集阶段的操作温度控制在590、620、650和680°C,同时将释放CO 2的温度保持在850°C。的CO的持续时间2的捕获阶段是3小时,使CO的2释放阶段为2小时。还研究了在650°C的碳化温度下吸附剂材料的碳化-煅烧循环的循环的影响。实验结果表明,在碳化过程的最初20分钟内,吸附剂材料有效捕获了CO 2。在煅烧阶段,从煅烧开始,CO 2释放速率在30-40分钟内达到峰值。碳酸化温度对CO 2的捕集有显着影响,最佳碳酸化温度为620°C,在该温度下,第一循环的CO 2捕集效率为94%,前三个循环的平均值为90.4%。还发现CO 2捕获效率随循环而降低。还研究了CO 2捕获的机理和循环的影响。
更新日期:2020-10-15
中文翻译:

使用新型钙和铁基吸收剂通过碳化-煅烧循环从生物质气化生产气中捕获CO 2
在这项研究中,研究了一种基于CaO和Fe 2 O 3的新型吸附剂材料在模拟生物质气化生产气中捕获CO 2中的性能。实验是在固定床反应器中进行的,实验的每轮操作都包括吸附剂还原,CO 2捕集(碳酸化)和CO 2释放(煅烧)三个主要阶段。将CO 2捕集阶段的操作温度控制在590、620、650和680°C,同时将释放CO 2的温度保持在850°C。的CO的持续时间2的捕获阶段是3小时,使CO的2释放阶段为2小时。还研究了在650°C的碳化温度下吸附剂材料的碳化-煅烧循环的循环的影响。实验结果表明,在碳化过程的最初20分钟内,吸附剂材料有效捕获了CO 2。在煅烧阶段,从煅烧开始,CO 2释放速率在30-40分钟内达到峰值。碳酸化温度对CO 2的捕集有显着影响,最佳碳酸化温度为620°C,在该温度下,第一循环的CO 2捕集效率为94%,前三个循环的平均值为90.4%。还发现CO 2捕获效率随循环而降低。还研究了CO 2捕获的机理和循环的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号