当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermophilic Pyrrolysyl-tRNA Synthetase Mutants for Enhanced Mammalian Genetic Code Expansion.
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-09-15 , DOI: 10.1021/acssynbio.0c00257 Liming Hu 1 , Xuewen Qin 1 , Yujia Huang 1 , Wenbing Cao 1, 2 , Chuchen Wang 1 , Yong Wang 1 , Xinyu Ling 1 , Heqi Chen 1 , Dan Wu 1 , Yu Lin 1 , Tao Liu 1
ACS Synthetic Biology ( IF 3.7 ) Pub Date : 2020-09-15 , DOI: 10.1021/acssynbio.0c00257 Liming Hu 1 , Xuewen Qin 1 , Yujia Huang 1 , Wenbing Cao 1, 2 , Chuchen Wang 1 , Yong Wang 1 , Xinyu Ling 1 , Heqi Chen 1 , Dan Wu 1 , Yu Lin 1 , Tao Liu 1
Affiliation
|
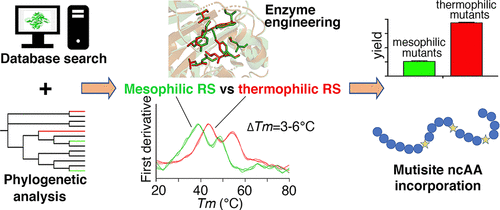
Genetic code expansion (GCE) is a powerful technique for site-specific incorporation of noncanonical amino acids (ncAAs) into proteins in living cells, which is achieved through evolved aminoacyl-tRNA synthetase mutants. Stability is important for promoting enzyme evolution, and we found that many of the evolved synthetase mutants have reduced thermostabilities. In this study, we characterized two novel pyrrolysyl-tRNA synthetases (PylRSs) derived from thermophilic archaea: Methanosarcina thermophila (Mt) and Methanosarcina flavescens (Mf). Further study demonstrated that the wild-type PylRSs and several mutants were orthogonal and active in both Escherichia coli and mammalian cells and could thus be used for GCE. Compared with the commonly used M. barkeri PylRS, the wild-type thermophilic PylRSs displayed reduced GCE efficiency; however, some of the mutants, as well as some chimeras, outperformed their mesophilic counterparts in mammalian cell culture at 37 °C. Their better performance could at least partially be attributed to the fact that these thermophilic synthetases exhibit a threshold of enhanced stability against destabilizing mutations to accommodate structurally diverse substrate analogues. These were indicated by the higher melting temperatures (by 3–6 °C) and the higher expression levels that were typically observed for the MtPylRS and MfPylRS mutants relative to the Mb equivalents. Using histone H3 as an example, we demonstrated that one of the thermophilic synthetase mutants promoted the incorporation of multiple acetyl-lysine residues in mammalian cells. The enzymes developed in this study add to the PylRS toolbox and provide potentially better scaffolds for PylRS engineering and evolution, which will be necessary to meet the increasing demands for expanded substrate repertoire with better efficiency and specificity in mammalian systems.
中文翻译:

用于增强哺乳动物遗传密码扩展的嗜热 Pyrrolysyl-tRNA 合成酶突变体。
遗传密码扩展 (GCE) 是一种强大的技术,可将非规范氨基酸 (ncAAs) 定点掺入活细胞中的蛋白质中,这是通过进化的氨酰-tRNA 合成酶突变体实现的。稳定性对于促进酶进化很重要,我们发现许多进化的合成酶突变体的热稳定性降低。在这项研究中,我们表征了两种源自嗜热古细菌的新型吡咯赖氨酰 tRNA 合成酶 (PylRS):嗜热甲烷八叠球菌( Mt ) 和黄蜡甲烷八叠球菌( Mf )。进一步的研究表明,野生型 PylRSs 和几个突变体在两种大肠杆菌中都是正交的和有活性的和哺乳动物细胞,因此可用于 GCE。与常用的M. barkeri PylRS 相比,野生型嗜热 PylRS 的 GCE 效率降低;然而,一些突变体以及一些嵌合体在 37°C 的哺乳动物细胞培养中表现优于其嗜温对应物。它们更好的性能至少部分归因于这样一个事实,即这些嗜热合成酶表现出针对不稳定突变的稳定性增强的阈值,以适应结构不同的底物类似物。这些由更高的解链温度(3-6°C)和更高的表达水平表明,这些表达水平通常在Mt PylRS 和Mf PylRS 突变体中观察到,相对于Mb等价物。以组蛋白 H3 为例,我们证明了一种嗜热合成酶突变体促进了哺乳动物细胞中多个乙酰赖氨酸残基的掺入。本研究中开发的酶添加到 PylRS 工具箱中,并为 PylRS 工程和进化提供了可能更好的支架,这对于满足在哺乳动物系统中具有更高效率和特异性的扩展底物库的日益增长的需求是必要的。
更新日期:2020-10-17
中文翻译:

用于增强哺乳动物遗传密码扩展的嗜热 Pyrrolysyl-tRNA 合成酶突变体。
遗传密码扩展 (GCE) 是一种强大的技术,可将非规范氨基酸 (ncAAs) 定点掺入活细胞中的蛋白质中,这是通过进化的氨酰-tRNA 合成酶突变体实现的。稳定性对于促进酶进化很重要,我们发现许多进化的合成酶突变体的热稳定性降低。在这项研究中,我们表征了两种源自嗜热古细菌的新型吡咯赖氨酰 tRNA 合成酶 (PylRS):嗜热甲烷八叠球菌( Mt ) 和黄蜡甲烷八叠球菌( Mf )。进一步的研究表明,野生型 PylRSs 和几个突变体在两种大肠杆菌中都是正交的和有活性的和哺乳动物细胞,因此可用于 GCE。与常用的M. barkeri PylRS 相比,野生型嗜热 PylRS 的 GCE 效率降低;然而,一些突变体以及一些嵌合体在 37°C 的哺乳动物细胞培养中表现优于其嗜温对应物。它们更好的性能至少部分归因于这样一个事实,即这些嗜热合成酶表现出针对不稳定突变的稳定性增强的阈值,以适应结构不同的底物类似物。这些由更高的解链温度(3-6°C)和更高的表达水平表明,这些表达水平通常在Mt PylRS 和Mf PylRS 突变体中观察到,相对于Mb等价物。以组蛋白 H3 为例,我们证明了一种嗜热合成酶突变体促进了哺乳动物细胞中多个乙酰赖氨酸残基的掺入。本研究中开发的酶添加到 PylRS 工具箱中,并为 PylRS 工程和进化提供了可能更好的支架,这对于满足在哺乳动物系统中具有更高效率和特异性的扩展底物库的日益增长的需求是必要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号