当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substituted ketenes offer exceptional carbon bases in gas phase: Computational study by density functional theory method
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-09-15 , DOI: 10.1002/poc.4142 Fariba Golpayegani 1 , Zohreh Mirjafary 1 , Hamid Saeidian 2 , Javad Mokhtari 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-09-15 , DOI: 10.1002/poc.4142 Fariba Golpayegani 1 , Zohreh Mirjafary 1 , Hamid Saeidian 2 , Javad Mokhtari 1
Affiliation
|
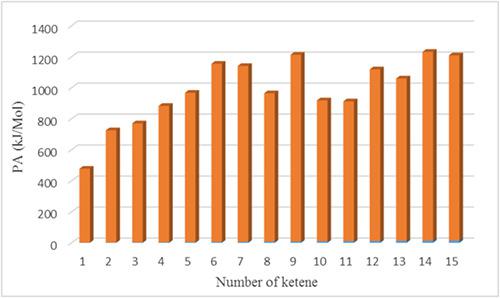
In the present study, ketene derivatives with cyclopropene and methylenecyclopropene substituents have been investigated by B3LYP/6‐311+G(d,p) level of theory to evaluate their suitability as powerful carbon superbases. The protonation at C (sp) site provides new neutral organic bases with proton affinities (PAs) = 879–1,218 kJ/mol, in which some are more basic than 1,8‐bis(dimethylamino)‐naphthalene, whose gas‐phase PA of 1,028 kJ/mol is considered the threshold of superbasicity. The PAs of designed ketenes were amplified by substitution of electron‐releasing groups such as methyl and dimethylamino on the molecular framework. Two indices of aromaticity, nucleus‐independent chemical shift and harmonic oscillator model of aromaticity, were also used for elucidation of ketene basicity, which reveals that a cyclopropene ring on the proposed ketene derivatives becomes aromatic upon protonation.
中文翻译:

取代的烯酮在气相中可提供优异的碳碱:通过密度泛函理论方法进行的计算研究
在本研究中,已通过B3LYP / 6-311 + G(d,p)的理论水平研究了具有环丙烯和亚甲基环丙烯取代基的乙烯酮衍生物,以评估其作为强力碳超碱的适用性。C(sp)位置的质子化提供了新的中性有机碱,质子亲和力(PAs)= 879-1,218 kJ / mol,其中一些碱比1,8-双(二甲基氨基)-萘(气相PA)更碱性1,028 kJ / mol的阈值被认为是超碱性的阈值。通过在分子框架上取代电子释放基团(例如甲基和二甲基氨基),可以扩增设计的乙烯酮的PA。还使用了两个芳香性指标,即与核无关的化学位移和芳香性的谐波振荡器模型来阐明乙烯酮的碱性,
更新日期:2020-09-15
中文翻译:

取代的烯酮在气相中可提供优异的碳碱:通过密度泛函理论方法进行的计算研究
在本研究中,已通过B3LYP / 6-311 + G(d,p)的理论水平研究了具有环丙烯和亚甲基环丙烯取代基的乙烯酮衍生物,以评估其作为强力碳超碱的适用性。C(sp)位置的质子化提供了新的中性有机碱,质子亲和力(PAs)= 879-1,218 kJ / mol,其中一些碱比1,8-双(二甲基氨基)-萘(气相PA)更碱性1,028 kJ / mol的阈值被认为是超碱性的阈值。通过在分子框架上取代电子释放基团(例如甲基和二甲基氨基),可以扩增设计的乙烯酮的PA。还使用了两个芳香性指标,即与核无关的化学位移和芳香性的谐波振荡器模型来阐明乙烯酮的碱性,











































 京公网安备 11010802027423号
京公网安备 11010802027423号