当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Visible‐Light Catalyzed [1+2+2] Cycloaddition Reactions Enabled by the Formation of Methylene Nitrones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/adsc.202000858 Jing Guo 1 , Ying Xie 1 , Wen‐Tian Zeng 1 , Qiao‐Lei Wu 1 , Jiang Weng 1 , Gui Lu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-09-16 , DOI: 10.1002/adsc.202000858 Jing Guo 1 , Ying Xie 1 , Wen‐Tian Zeng 1 , Qiao‐Lei Wu 1 , Jiang Weng 1 , Gui Lu 1
Affiliation
|
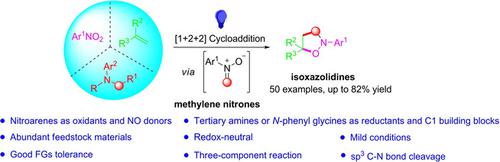
Nitrones are key intermediates in organic synthesis. Herein, we report the first photo‐redox synthesis of methylene nitrone intermediates from nitroarenes and arylamines. The highly reactive methylene nitrones are in situ trapped by alkenes to afford various isoxazolidines. This three‐component reaction features the use of N,N‐dimethylanilines or N‐aryl glycines as C1 building blocks, which allow for the one‐pot formal [1+2+2] cycloaddition from simple starting materials. A wide range of useful isoxazolidines can be obtained under mild conditions with moderate to good yields. Mechanistic investigations support the formation of methylene nitrone via selective N−CH3 bond cleavage and methylene transfer.
中文翻译:

可见光催化的亚硝基形成形成的[1 + 2 + 2]环加成反应
硝基是有机合成中的关键中间体。本文中,我们报道了由硝基芳烃和芳基胺进行的光催化还原亚甲基中间体中间体。高反应性的亚甲基硝基酮被烯烃原位捕获,得到各种异恶唑烷。此三组分反应的特征是使用N,N-二甲基苯胺或N-芳基甘氨酸作为C1结构单元,可从简单的起始原料进行一锅形式的正式[1 + 2 + 2]环加成。在温和的条件下,可以以中等至良好的收率获得各种有用的异恶唑烷。机理研究支持通过选择性的N-CH 3键裂解和亚甲基转移形成亚甲基硝酮。
更新日期:2020-09-16
中文翻译:

可见光催化的亚硝基形成形成的[1 + 2 + 2]环加成反应
硝基是有机合成中的关键中间体。本文中,我们报道了由硝基芳烃和芳基胺进行的光催化还原亚甲基中间体中间体。高反应性的亚甲基硝基酮被烯烃原位捕获,得到各种异恶唑烷。此三组分反应的特征是使用N,N-二甲基苯胺或N-芳基甘氨酸作为C1结构单元,可从简单的起始原料进行一锅形式的正式[1 + 2 + 2]环加成。在温和的条件下,可以以中等至良好的收率获得各种有用的异恶唑烷。机理研究支持通过选择性的N-CH 3键裂解和亚甲基转移形成亚甲基硝酮。









































 京公网安备 11010802027423号
京公网安备 11010802027423号