当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coating of a Novel Antimicrobial Nanoparticle with a Macrophage Membrane for the Selective Entry into Infected Macrophages and Killing of Intracellular Staphylococci
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-09-16 , DOI: 10.1002/adfm.202004942 Yuanfeng Li 1, 2 , Yong Liu 1, 2 , Yijin Ren 3 , Linzhu Su 1 , Ang Li 1 , Yingli An 1 , Vincent Rotello 4 , Zhanzhan Zhang 1 , Yin Wang 1 , Yang Liu 1 , Sidi Liu 5 , Jian Liu 5 , Jon D Laman 6 , Linqi Shi 1 , Henny C van der Mei 2 , Henk J Busscher 2
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2020-09-16 , DOI: 10.1002/adfm.202004942 Yuanfeng Li 1, 2 , Yong Liu 1, 2 , Yijin Ren 3 , Linzhu Su 1 , Ang Li 1 , Yingli An 1 , Vincent Rotello 4 , Zhanzhan Zhang 1 , Yin Wang 1 , Yang Liu 1 , Sidi Liu 5 , Jian Liu 5 , Jon D Laman 6 , Linqi Shi 1 , Henny C van der Mei 2 , Henk J Busscher 2
Affiliation
|
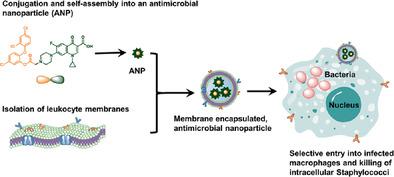
Internalization of Staphylococcus aureus by macrophages can inactivate bacterial killing mechanisms, allowing intracellular residence and dissemination of infection. Concurrently, these staphylococci can evade antibiotics that are frequently unable to pass mammalian cell membranes. A binary, amphiphilic conjugate composed of triclosan and ciprofloxacin is synthesized that self‐assemble through micelle formation into antimicrobial nanoparticles (ANPs). These novel ANPs are stabilized through encapsulation in macrophage membranes, providing membrane‐encapsulated, antimicrobial‐conjugated NPs (Me‐ANPs) with similar protein activity, Toll‐like receptor expression and negative surface charge as their precursor murine macrophage/human monocyte cell lines. The combination of Toll‐like receptors and negative surface charge allows uptake of Me‐ANPs by infected macrophages/monocytes through positively charged, lysozyme‐rich membrane scars created during staphylococcal engulfment. Me‐ANPs are not engulfed by more negatively charged sterile cells possessing less lysozyme at their surface. The Me‐ANPs kill staphylococci internalized in macrophages in vitro. Me‐ANPs likewise kill staphylococci more effectively than ANPs without membrane‐encapsulation or clinically used ciprofloxacin in a mouse peritoneal infection model. Similarly, organ infections in mice created by dissemination of infected macrophages through circulation in the blood are better eradicated by Me‐ANPs than by ciprofloxacin. These unique antimicrobial properties of macrophage‐monocyte Me‐ANPs provide a promising direction for human clinical application to combat persistent infections.
中文翻译:

用巨噬细胞膜包覆新型抗菌纳米颗粒,用于选择性进入受感染的巨噬细胞并杀死细胞内葡萄球菌
内在化的金黄色葡萄球菌巨噬细胞可以使细菌杀伤机制失活,从而允许细胞内驻留和感染传播。同时,这些葡萄球菌可以避开通常无法通过哺乳动物细胞膜的抗生素。合成了一种由三氯生和环丙沙星组成的二元两亲共轭物,通过胶束形成自组装成抗菌纳米粒子 (ANPs)。这些新型 ANPs 通过封装在巨噬细胞膜中而稳定,提供具有类似蛋白质活性、Toll 样受体表达和负表面电荷的膜封装、抗菌缀合 NPs(Me-ANPs)作为它们的前体鼠巨噬细胞/人单核细胞系。Toll 样受体和负表面电荷的组合允许受感染的巨噬细胞/单核细胞通过葡萄球菌吞噬过程中产生的带正电荷、富含溶菌酶的膜疤痕吸收 Me-ANP。Me-ANP 不会被表面具有较少溶菌酶的带负电的无菌细胞吞噬。Me-ANPs 在体外杀死巨噬细胞中内化的葡萄球菌。在小鼠腹膜感染模型中,Me-ANPs 同样比没有膜包裹的 ANPs 或临床使用的环丙沙星更有效地杀死葡萄球菌。同样,Me-ANPs 比环丙沙星更能根除由受感染的巨噬细胞通过血液循环传播而引起的小鼠器官感染。
更新日期:2020-11-25
中文翻译:

用巨噬细胞膜包覆新型抗菌纳米颗粒,用于选择性进入受感染的巨噬细胞并杀死细胞内葡萄球菌
内在化的金黄色葡萄球菌巨噬细胞可以使细菌杀伤机制失活,从而允许细胞内驻留和感染传播。同时,这些葡萄球菌可以避开通常无法通过哺乳动物细胞膜的抗生素。合成了一种由三氯生和环丙沙星组成的二元两亲共轭物,通过胶束形成自组装成抗菌纳米粒子 (ANPs)。这些新型 ANPs 通过封装在巨噬细胞膜中而稳定,提供具有类似蛋白质活性、Toll 样受体表达和负表面电荷的膜封装、抗菌缀合 NPs(Me-ANPs)作为它们的前体鼠巨噬细胞/人单核细胞系。Toll 样受体和负表面电荷的组合允许受感染的巨噬细胞/单核细胞通过葡萄球菌吞噬过程中产生的带正电荷、富含溶菌酶的膜疤痕吸收 Me-ANP。Me-ANP 不会被表面具有较少溶菌酶的带负电的无菌细胞吞噬。Me-ANPs 在体外杀死巨噬细胞中内化的葡萄球菌。在小鼠腹膜感染模型中,Me-ANPs 同样比没有膜包裹的 ANPs 或临床使用的环丙沙星更有效地杀死葡萄球菌。同样,Me-ANPs 比环丙沙星更能根除由受感染的巨噬细胞通过血液循环传播而引起的小鼠器官感染。











































 京公网安备 11010802027423号
京公网安备 11010802027423号