当前位置:
X-MOL 学术
›
Process Saf. Environ. Prot.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superior reduction of nitrate with simultaneous oxidation of intermediates and enhanced nitrogen gas selectivity via novel electrochemical treatment
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.psep.2020.09.026 Rohit Chauhan , Vimal Chandra Srivastava
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.psep.2020.09.026 Rohit Chauhan , Vimal Chandra Srivastava
|
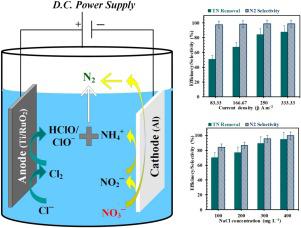
Abstract This study reports an electrochemical reduction of the NO3− along with oxidation of the in-situ generated NH4+ with maximum selectivity of the N2 gas as the final-product. The use of aluminum as a cathode and Ti/RuO2 as an anode showed enhanced electrochemical nitrate reduction at the cathode and oxidation of the ammonium ion at the anode. Effects of various parameters like initial NO3− concentration (Co = 100−400 mg L−1), a dose of the Cl− as NaCl (NaCl = 100−400 mg L−1), current density applied (j = 83.3-333.3 A m−2), solution pH (pH = 4–10) and electrolysis time (t = 0−120 min) were studied in terms of NO3− reduction and total nitrogen (TN) removal efficiencies. Current efficiency (CE) was elaborated with respect to end products like N2, NO2− and NH4+. Specific electrical energy consumption (SEC) was calculated in kWh kg−1 NO3− removed for the electrochemical process. Electrochemical impedance spectroscopy (EIS) and cyclic voltammetry (CV) were utilized for understanding the oxidation/reduction mechanism over electrodes and the characteristics of the electrodes in a different solution. The studied mechanism suggested a circular conversion of NO3− through complex processes into the N2 gas as the final product. The ultimate nitrate and TN degradation efficiency of ≈95 % with N2 selectivity of ≈100 % were achieved at the optimum condition of Co = 100 mg L−1, NaCl = 300 mg L−1, j = 333.3 A m−2, pH = 6 and time = 120 min with SEC = 927.4 kW h kg−1 NO3− removed. The 1st, 2nd, and nth-order kinetic models were used for the reaction kinetics. FE-SEM, XRD, and AFM techniques were used for the characterization of the electrodes before and after all the electrochemical runs. The operating cost was calculated for lab-scale treatment along with a comparison with previous studies. No sludge or scum got produced for each electrochemical run. Finally, this study delivers a superior perceptive for electrochemical characteristics of Al at the cathode side and Ti/RuO2 at anode side as well as electrochemical NO3− reduction and oxidation of the generated NH4+, simultaneously.
中文翻译:

通过新型电化学处理在中间体氧化的同时实现硝酸盐的出色还原和提高氮气选择性
摘要 本研究报告了 NO3- 的电化学还原以及原位生成的 NH4+ 的氧化,其中 N2 气体作为最终产物的选择性最大。使用铝作为阴极和使用 Ti/RuO2 作为阳极显示出增强的阴极电化学硝酸盐还原和阳极铵离子的氧化。各种参数的影响,如初始 NO3− 浓度(Co = 100−400 mg L−1)、Cl− 作为 NaCl 的剂量(NaCl = 100−400 mg L−1)、施加的电流密度(j = 83.3-333.3根据 NO3− 还原和总氮 (TN) 去除效率研究了 m-2)、溶液 pH(pH = 4-10)和电解时间(t = 0-120 分钟)。针对 N2、NO2- 和 NH4+ 等最终产品详细阐述了电流效率 (CE)。比电能消耗 (SEC) 以 kWh kg-1 NO3- 为单位计算,用于电化学过程。电化学阻抗谱 (EIS) 和循环伏安法 (CV) 用于了解电极上的氧化/还原机制以及电极在不同溶液中的特性。研究的机制表明 NO3− 通过复杂的过程循环转化为 N2 气作为最终产品。在 Co = 100 mg L−1、NaCl = 300 mg L−1、j = 333.3 A m−2、pH 的最佳条件下,最终硝酸盐和 TN 降解效率 ≈95%,N2 选择性 ≈100% = 6 和时间 = 120 分钟,去除 SEC = 927.4 kW h kg−1 NO3−。一级、二级和三级动力学模型用于反应动力学。FE-SEM、XRD、和 AFM 技术用于在所有电化学运行之前和之后表征电极。计算实验室规模处理的运营成本,并与之前的研究进行比较。每次电化学运行都没有产生污泥或浮渣。最后,这项研究对阴极侧的 Al 和阳极侧的 Ti/RuO2 的电化学特性以及生成的 NH4+ 的电化学 NO3- 还原和氧化提供了卓越的感知。
更新日期:2021-03-01
中文翻译:

通过新型电化学处理在中间体氧化的同时实现硝酸盐的出色还原和提高氮气选择性
摘要 本研究报告了 NO3- 的电化学还原以及原位生成的 NH4+ 的氧化,其中 N2 气体作为最终产物的选择性最大。使用铝作为阴极和使用 Ti/RuO2 作为阳极显示出增强的阴极电化学硝酸盐还原和阳极铵离子的氧化。各种参数的影响,如初始 NO3− 浓度(Co = 100−400 mg L−1)、Cl− 作为 NaCl 的剂量(NaCl = 100−400 mg L−1)、施加的电流密度(j = 83.3-333.3根据 NO3− 还原和总氮 (TN) 去除效率研究了 m-2)、溶液 pH(pH = 4-10)和电解时间(t = 0-120 分钟)。针对 N2、NO2- 和 NH4+ 等最终产品详细阐述了电流效率 (CE)。比电能消耗 (SEC) 以 kWh kg-1 NO3- 为单位计算,用于电化学过程。电化学阻抗谱 (EIS) 和循环伏安法 (CV) 用于了解电极上的氧化/还原机制以及电极在不同溶液中的特性。研究的机制表明 NO3− 通过复杂的过程循环转化为 N2 气作为最终产品。在 Co = 100 mg L−1、NaCl = 300 mg L−1、j = 333.3 A m−2、pH 的最佳条件下,最终硝酸盐和 TN 降解效率 ≈95%,N2 选择性 ≈100% = 6 和时间 = 120 分钟,去除 SEC = 927.4 kW h kg−1 NO3−。一级、二级和三级动力学模型用于反应动力学。FE-SEM、XRD、和 AFM 技术用于在所有电化学运行之前和之后表征电极。计算实验室规模处理的运营成本,并与之前的研究进行比较。每次电化学运行都没有产生污泥或浮渣。最后,这项研究对阴极侧的 Al 和阳极侧的 Ti/RuO2 的电化学特性以及生成的 NH4+ 的电化学 NO3- 还原和氧化提供了卓越的感知。











































 京公网安备 11010802027423号
京公网安备 11010802027423号