European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-09-16 , DOI: 10.1016/j.ejmech.2020.112846 Huabin Hu , Jürgen Bajorath
|
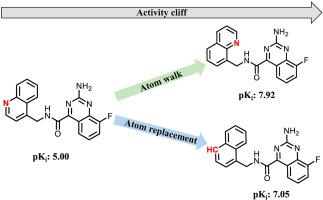
In medicinal chemistry, activity cliffs (ACs) are considered as sources of critical structure-activity relationship (SAR) information. ACs are capable of revealing such SAR information because they are formed by pairs or groups of structural analogs that are distinguished by small chemical modifications leading to large variations in compound potency. Such modifications can reveal critically important substitution sites in analog series. Small AC-encoded chemical changes enable the identification of SAR determinants. In this work, we have searched medicinal chemistry data for most “subtle” ACs in which participating compounds are only distinguished by single-atom modifications. These ACs can be directly associated with lead optimization strategies such as positional atom scanning (atom “walks”) or heteroatom replacements in ring structures. More than 1500 of these ACs with activity against a variety of targets were identified. To further explore newly identified ACs, we searched for X-ray structures of ligand-target complexes containing participating AC compounds. For a subset of subtle ACs, X-ray structures of complexes made it possible to examine effects of single-atom changes in light of well-defined ligand-target interactions. Since ACs capturing minimal chemical changes are of particular interest for lead optimization and drug design, we make all newly identified ACs and associated structural information freely available as an open access deposition.
中文翻译:

活性化合物的单原子修饰产生的活性悬崖:基于X射线结构的系统识别和合理化
在药物化学中,活性悬崖(ACs)被视为关键结构-活性关系(SAR)信息的来源。AC能够显示此类SAR信息,因为它们是由成对或成对的结构类似物组成的,这些类似物的化学修饰很小,导致化合物效价发生较大变化。此类修饰可以揭示类似物系列中至关重要的取代位点。AC编码的微小化学变化使您能够识别SAR决定因素。在这项工作中,我们搜索了大多数“细微” AC的药物化学数据,在这些AC中,参与化合物仅通过单原子修饰来区分。这些AC可以直接与铅优化策略相关联,例如位置原子扫描(原子“行走”)或环结构中的杂原子替代。这些AC中有1500多个具有针对多种目标的活性。为了进一步探索新发现的AC,我们搜索了包含参与AC化合物的配体-目标配合物的X射线结构。对于细微AC的子集,复合物的X射线结构使得可以根据定义明确的配体与靶标相互作用来检查单原子变化的影响。由于捕获最小化学变化的AC对于铅优化和药物设计尤为重要,因此我们将所有新识别的AC和相关的结构信息作为开放存取沉积物免费提供。对于细微的AC的子集,复合物的X射线结构使得可以根据定义明确的配体-靶标相互作用研究单原子变化的影响。由于捕获最小化学变化的AC对于铅优化和药物设计尤为重要,因此我们将所有新识别的AC和相关的结构信息作为开放存取沉积物免费提供。对于细微AC的子集,复合物的X射线结构使得可以根据定义明确的配体与靶标相互作用来检查单原子变化的影响。由于捕获最少量化学变化的AC对于铅优化和药物设计尤为重要,因此我们将所有新识别的AC及其相关的结构信息作为开放存取沉积物免费提供。











































 京公网安备 11010802027423号
京公网安备 11010802027423号