Ecotoxicology and Environmental Safety ( IF 6.2 ) Pub Date : 2020-09-15 , DOI: 10.1016/j.ecoenv.2020.111280 Xiao Su 1 , Leng Wang 1 , Yefei Xu 1 , Lili Dong 1 , Huizhe Lu 1
|
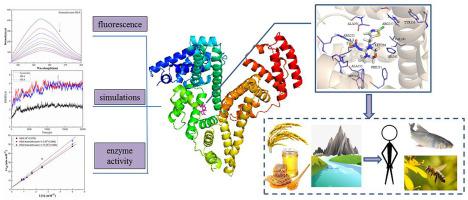
As a top-selling neonicotinoid insecticide widely used in the field, thiamethoxam is an environmental pollutant because of the accumulation in ecosystem and has also been reported that it has potential risks to the health of mammals even humans. In order to understand the binding mechanism of thiamethoxam with biological receptors, spectroscopic techniques and theoretical simulations was used to explore the specific interactions between thiamethoxam and proteins. Interestingly, the results indicated that hydrophobic interaction as the main driving force, thiamethoxam formed a single binding site complex with proteins spontaneously, resulting in a decrease in the esterase-like activity of human serum albumin. The results of computer simulation showed that there were hydrophobic, electrostatic and hydrogen bonding interactions between thiamethoxam and receptors. The results of experiment and computer simulation were mutually confirmed, so a model was established for the interaction between the two which uncovered the structural characteristics of the binding site. This research provided new insights for the structure optimization of thiamethoxam, as well as gave an effective reference for evaluating the risk of thiamethoxam systemically in the future.
中文翻译:

噻虫嗪与三种模型蛋白的结合机理研究:光谱学和理论模拟。
作为在该领域广泛使用的最畅销的新烟碱类杀虫剂,噻虫嗪由于在生态系统中的积累而成为一种环境污染物,并且据报道,它对哺乳动物甚至人类的健康都具有潜在的风险。为了了解噻虫嗪与生物受体的结合机理,采用光谱技术和理论模拟研究了噻虫嗪与蛋白质之间的特异性相互作用。有趣的是,结果表明,以疏水相互作用为主要驱动力,噻虫嗪自发形成与蛋白质的单个结合位点复合物,导致人血清白蛋白的酯酶样活性降低。计算机仿真结果表明存在疏水性,噻虫嗪与受体之间的静电和氢键相互作用。实验和计算机仿真的结果是相互证实的,因此为两者之间的相互作用建立了一个模型,从而揭示了结合位点的结构特征。该研究为噻虫嗪的结构优化提供了新的见识,并为今后系统评价噻虫嗪的风险提供了有效的参考。










































 京公网安备 11010802027423号
京公网安备 11010802027423号