Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Graphene oxide sheets and quantum dots inhibit α-synuclein amyloid formation by different mechanisms.
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-15 , DOI: 10.1039/d0nr05003b Marziyeh Ghaeidamini 1 , David Bernson 1 , Nima Sasanian 1 , Ranjeet Kumar 1 , Elin K Esbjörner 1
Nanoscale ( IF 5.8 ) Pub Date : 2020-09-15 , DOI: 10.1039/d0nr05003b Marziyeh Ghaeidamini 1 , David Bernson 1 , Nima Sasanian 1 , Ranjeet Kumar 1 , Elin K Esbjörner 1
Affiliation
|
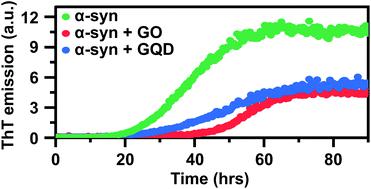
Aggregation and amyloid formation of the 140-residue presynaptic and intrinsically disordered protein α-synuclein (α-syn) is a pathological hallmark of Parkinson's disease (PD). Understanding how α-syn forms amyloid fibrils, and investigations of agents that can prevent their formation is therefore important. We demonstrate herein that two types of graphene oxide nanoparticles (sheets and quantum dots) inhibit α-syn amyloid formation by different mechanisms mediated via differential interactions with both monomers and fibrils. We have used thioflavin-T fluorescence assays and kinetic analysis, circular dichroism, dynamic light scattering, fluorescence spectroscopy and atomic force microscopy to asses the kinetic nature and efficiency of this inhibitory effect. We show that the two types of graphene oxide nanoparticles alter the morphology of α-syn fibrils, disrupting their interfilament assembly and the resulting aggregates therefore consist of single protofilaments. Our results further show that graphene oxide sheets reduce the aggregation rate of α-syn primarily by sequestering of monomers, thereby preventing primary nucleation and elongation. Graphene quantum dots, on the other hand, interact less avidly with both monomers and fibrils. Their aggregation inhibitory effect is primarily related to adsorption of aggregated species and reduction of secondary processes, and they can thus not fully prevent aggregation. This fine-tuned and differential effect of graphene nanoparticles on amyloid formation shows that rational design of these nanomaterials has great potential in engineering materials that interact with specific molecular events in the amyloid fibril formation process. The findings also provide new insight into the molecular interplay between amyloidogenic proteins and graphene-based nanomaterials in general, and opens up their potential use as agents to manipulate fibril formation.
中文翻译:

氧化石墨烯片和量子点通过不同的机制抑制α-突触核蛋白淀粉样蛋白的形成。
140个残基的突触前和固有紊乱的蛋白质α-突触核蛋白(α-syn)的聚集和淀粉样蛋白形成是帕金森氏病(PD)的病理标志。因此,了解α-syn如何形成淀粉样蛋白原纤维以及研究可防止其形成的物质非常重要。我们在此表明,两种类型的氧化石墨烯纳米颗粒(片材和量子点)抑制α-syn时淀粉样蛋白形成的通过不同的机制介导的经由与单体和原纤维的微分相互作用。我们已经使用硫代黄素-T荧光测定和动力学分析,圆二色性,动态光散射,荧光光谱和原子力显微镜来评估这种抑制作用的动力学性质和效率。我们表明,两种类型的氧化石墨烯纳米颗粒改变了α-syn原纤维的形态,破坏了它们的纤丝间的组装,因此产生的聚集体因此由单个原丝组成。我们的结果进一步表明,氧化石墨烯片主要通过螯合单体来降低α-syn的聚集速率,从而防止了初级成核和伸长。另一方面,石墨烯量子点与单体和原纤维的相互作用较小。它们的聚集抑制作用主要与聚集物质的吸附和次级过程的减少有关,因此它们不能完全防止聚集。石墨烯纳米颗粒对淀粉样蛋白形成的微调和微分作用表明,这些纳米材料的合理设计在与淀粉样蛋白原纤维形成过程中与特定分子事件相互作用的工程材料中具有巨大潜力。这些发现还为淀粉样蛋白和基于石墨烯的纳米材料之间的分子相互作用提供了新的见解,并开辟了它们作为操纵原纤维形成的剂的潜在用途。石墨烯纳米颗粒对淀粉样蛋白形成的微调和差异作用表明,这些纳米材料的合理设计在与淀粉样蛋白原纤维形成过程中与特定分子事件相互作用的工程材料中具有巨大潜力。这些发现还为淀粉样蛋白和基于石墨烯的纳米材料之间的分子相互作用提供了新的见解,并开辟了它们作为操纵原纤维形成的剂的潜在用途。石墨烯纳米颗粒对淀粉样蛋白形成的微调和微分作用表明,这些纳米材料的合理设计在与淀粉样蛋白原纤维形成过程中与特定分子事件相互作用的工程材料中具有巨大潜力。这些发现也为淀粉样蛋白和基于石墨烯的纳米材料之间的分子相互作用提供了新的见解,并开辟了它们作为操纵原纤维形成的潜力。
更新日期:2020-10-02
中文翻译:

氧化石墨烯片和量子点通过不同的机制抑制α-突触核蛋白淀粉样蛋白的形成。
140个残基的突触前和固有紊乱的蛋白质α-突触核蛋白(α-syn)的聚集和淀粉样蛋白形成是帕金森氏病(PD)的病理标志。因此,了解α-syn如何形成淀粉样蛋白原纤维以及研究可防止其形成的物质非常重要。我们在此表明,两种类型的氧化石墨烯纳米颗粒(片材和量子点)抑制α-syn时淀粉样蛋白形成的通过不同的机制介导的经由与单体和原纤维的微分相互作用。我们已经使用硫代黄素-T荧光测定和动力学分析,圆二色性,动态光散射,荧光光谱和原子力显微镜来评估这种抑制作用的动力学性质和效率。我们表明,两种类型的氧化石墨烯纳米颗粒改变了α-syn原纤维的形态,破坏了它们的纤丝间的组装,因此产生的聚集体因此由单个原丝组成。我们的结果进一步表明,氧化石墨烯片主要通过螯合单体来降低α-syn的聚集速率,从而防止了初级成核和伸长。另一方面,石墨烯量子点与单体和原纤维的相互作用较小。它们的聚集抑制作用主要与聚集物质的吸附和次级过程的减少有关,因此它们不能完全防止聚集。石墨烯纳米颗粒对淀粉样蛋白形成的微调和微分作用表明,这些纳米材料的合理设计在与淀粉样蛋白原纤维形成过程中与特定分子事件相互作用的工程材料中具有巨大潜力。这些发现还为淀粉样蛋白和基于石墨烯的纳米材料之间的分子相互作用提供了新的见解,并开辟了它们作为操纵原纤维形成的剂的潜在用途。石墨烯纳米颗粒对淀粉样蛋白形成的微调和差异作用表明,这些纳米材料的合理设计在与淀粉样蛋白原纤维形成过程中与特定分子事件相互作用的工程材料中具有巨大潜力。这些发现还为淀粉样蛋白和基于石墨烯的纳米材料之间的分子相互作用提供了新的见解,并开辟了它们作为操纵原纤维形成的剂的潜在用途。石墨烯纳米颗粒对淀粉样蛋白形成的微调和微分作用表明,这些纳米材料的合理设计在与淀粉样蛋白原纤维形成过程中与特定分子事件相互作用的工程材料中具有巨大潜力。这些发现也为淀粉样蛋白和基于石墨烯的纳米材料之间的分子相互作用提供了新的见解,并开辟了它们作为操纵原纤维形成的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号