当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-mediated peptide arylation selective for the N-terminus
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-14 , DOI: 10.1039/d0sc02933e Mary K Miller 1 , Haopei Wang 1 , Kengo Hanaya 1 , Olivia Zhang 1 , Alex Berlaga 1 , Zachary T Ball 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-14 , DOI: 10.1039/d0sc02933e Mary K Miller 1 , Haopei Wang 1 , Kengo Hanaya 1 , Olivia Zhang 1 , Alex Berlaga 1 , Zachary T Ball 1
Affiliation
|
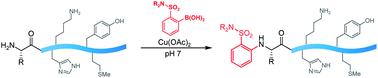
Polypeptides present remarkable selectivity challenges for chemical methods. Amino groups are ubiquitous in polypeptide structure, yet few paradigms exist for reactivity and selectivity in arylation of amine groups. This communication describes the utilization of boronic acid reagents bearing certain o-electron withdrawing groups for copper-mediated amine arylation of the N-terminus under mild conditions and primarily aqueous solvent. The method adds to the toolkit of boronic acid reagents for polypeptide modification under mild conditions in water that shows complete selectivity for the N-terminus in the presence of lysine side chains.
中文翻译:

铜介导的 N 末端选择性芳基化
多肽对化学方法提出了显着的选择性挑战。氨基在多肽结构中无处不在,但胺基芳基化的反应性和选择性的范例很少。该通讯描述了在温和条件和主要水溶剂下,利用带有某些邻电子吸电子基团的硼酸试剂进行铜介导的N末端胺芳基化。该方法添加到硼酸试剂工具包中,用于在温和条件下在水中进行多肽修饰,在赖氨酸侧链存在的情况下显示出对 N 末端的完全选择性。
更新日期:2020-10-07
中文翻译:

铜介导的 N 末端选择性芳基化
多肽对化学方法提出了显着的选择性挑战。氨基在多肽结构中无处不在,但胺基芳基化的反应性和选择性的范例很少。该通讯描述了在温和条件和主要水溶剂下,利用带有某些邻电子吸电子基团的硼酸试剂进行铜介导的N末端胺芳基化。该方法添加到硼酸试剂工具包中,用于在温和条件下在水中进行多肽修饰,在赖氨酸侧链存在的情况下显示出对 N 末端的完全选择性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号