当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triple Strategies to Improve Oral Bioavailability by Fabricating Co-amorphous Forms of Ursolic Acid with Piperine: Enhancing Water-Solubility, Permeability and Inhibiting Cytochrome P450 Isozymes.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-09-14 , DOI: 10.1021/acs.molpharmaceut.0c00443 Danni Yu 1 , Zigui Kan 1, 2 , Fei Shan 3 , Jing Zang 3 , Jianping Zhou 3
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-09-14 , DOI: 10.1021/acs.molpharmaceut.0c00443 Danni Yu 1 , Zigui Kan 1, 2 , Fei Shan 3 , Jing Zang 3 , Jianping Zhou 3
Affiliation
|
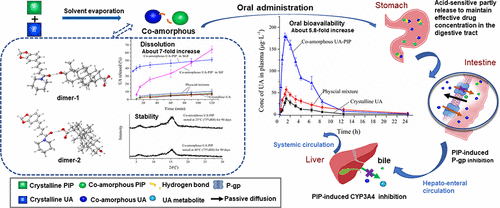
As a BCS IV drug, ursolic acid (UA) has low oral bioavailability mainly because of its poor aqueous solubility/dissolution, poor permeability, and metabolism by cytochrome P450 (CYP) isozymes, such as CYP3A4. Most UA preparations demonstrated a much higher dissolution than that of its crystalline form yet a low drug concentration in plasma due to their lower consideration or evaluation for the permeability and metabolism issues. In the current study, a supramolecular coamorphous system of UA with piperine (PIP) was prepared and characterized by powder X-ray diffraction, differential scanning calorimetry, and scanning electron microscopy. In comparison to crystalline UA and UA in physical mixture, such coamorphous system enhanced solubility (5.3–7-fold in the physiological solution) and dissolution (7–8-fold in the physiological solution within 2 h) of UA and exhibited excellent physical stability under 90-day storage conditions. More importantly, the pharmacokinetic study of coamorphous UA in rats exhibited 5.8-fold and 2.47-fold improvement in AUC0-∞ value, respectively, compared with its free and mixed crystalline counterparts. In order to further explore the mechanism of such improvement, the molecular interactions of a coamorphous system in the solid state were investigated. Fourier transform infrared spectroscopy, solid-state 13C nuclear magnetic resonance spectroscopy, and density functional theory modeling suggested that intermolecular hydrogen bonds with strong interactions newly formed between UA and PIP after coamorphization. The in vitro permeability studies across Caco-2 cell monolayer and metabolism studies by rat hepatic microsomes indicated that free PIP significantly increased the permeability of UA and inhibited the enzymatic metabolism of UA by CYP3A4. However, PIP in the coamorphous combination exhibited a much lower level in the bioenhancing than its free form arising from the synchronized dissolution characteristic of the preparation (only 60% of PIP released in comparison to its free counterpart in 2 h). The in situ loop study in rats proposed that the acid-sensitive dissolution in the stomach of the coamorphous preparation helped to improve the effective free drug concentration, thereby facilitating PIP to play its role in bioenhancing. The current study offers an exploratory strategy to overcome poor solubility/dissolution, poor permeability, and metabolism by cytochrome P450 isozymes of the BCS IV drug to improve its oral bioavailability.
中文翻译:

通过制备熊果酸与胡椒碱的共无定形形式来提高口服生物利用度的三重策略:增强水溶性、渗透性和抑制细胞色素 P450 同工酶。
作为BCS IV药物,熊果酸(UA)口服生物利用度较低,主要是因为其水溶性/溶出度差、渗透性差以及被细胞色素P450(CYP)同工酶(如CYP3A4)代谢。大多数UA制剂表现出比其晶型更高的溶出度,但由于对渗透性和代谢问题的考虑或评估较低,血浆中的药物浓度较低。在本研究中,制备了UA与胡椒碱(PIP)的超分子共晶体系,并通过粉末X射线衍射、差示扫描量热法和扫描电子显微镜进行了表征。与结晶UA和物理混合物中的UA相比,这种共晶体系提高了UA的溶解度(在生理溶液中5.3-7倍)和溶出度(2小时内在生理溶液中7-8倍),并表现出优异的物理稳定性在90天的储存条件下。更重要的是,与游离和混合结晶UA相比,共晶UA在大鼠中的药代动力学研究显示AUC 0-∞值分别提高了5.8倍和2.47倍。为了进一步探索这种改进的机制,研究了固态共非晶系统的分子相互作用。傅里叶变换红外光谱、固态13C核磁共振光谱和密度泛函理论模型表明,共晶化后UA和PIP之间新形成了具有强相互作用的分子间氢键。 Caco-2单层细胞的体外渗透性研究和大鼠肝微粒体的代谢研究表明,游离PIP显着增加UA的渗透性并抑制CYP3A4对UA的酶促代谢。然而,由于制剂的同步溶解特性,共晶组合中的 PIP 表现出比其游离形式低得多的生物增强水平(与游离形式相比,2 小时内仅释放了 60% 的 PIP)。大鼠原位循环研究表明,共晶制剂在胃中的酸敏感溶解有助于提高有效游离药物浓度,从而有利于PIP发挥其生物增强作用。目前的研究提供了一种探索性策略,克服 BCS IV 药物的溶解度/溶出度差、渗透性差以及细胞色素 P450 同工酶的代谢,以提高其口服生物利用度。
更新日期:2020-09-14
中文翻译:

通过制备熊果酸与胡椒碱的共无定形形式来提高口服生物利用度的三重策略:增强水溶性、渗透性和抑制细胞色素 P450 同工酶。
作为BCS IV药物,熊果酸(UA)口服生物利用度较低,主要是因为其水溶性/溶出度差、渗透性差以及被细胞色素P450(CYP)同工酶(如CYP3A4)代谢。大多数UA制剂表现出比其晶型更高的溶出度,但由于对渗透性和代谢问题的考虑或评估较低,血浆中的药物浓度较低。在本研究中,制备了UA与胡椒碱(PIP)的超分子共晶体系,并通过粉末X射线衍射、差示扫描量热法和扫描电子显微镜进行了表征。与结晶UA和物理混合物中的UA相比,这种共晶体系提高了UA的溶解度(在生理溶液中5.3-7倍)和溶出度(2小时内在生理溶液中7-8倍),并表现出优异的物理稳定性在90天的储存条件下。更重要的是,与游离和混合结晶UA相比,共晶UA在大鼠中的药代动力学研究显示AUC 0-∞值分别提高了5.8倍和2.47倍。为了进一步探索这种改进的机制,研究了固态共非晶系统的分子相互作用。傅里叶变换红外光谱、固态13C核磁共振光谱和密度泛函理论模型表明,共晶化后UA和PIP之间新形成了具有强相互作用的分子间氢键。 Caco-2单层细胞的体外渗透性研究和大鼠肝微粒体的代谢研究表明,游离PIP显着增加UA的渗透性并抑制CYP3A4对UA的酶促代谢。然而,由于制剂的同步溶解特性,共晶组合中的 PIP 表现出比其游离形式低得多的生物增强水平(与游离形式相比,2 小时内仅释放了 60% 的 PIP)。大鼠原位循环研究表明,共晶制剂在胃中的酸敏感溶解有助于提高有效游离药物浓度,从而有利于PIP发挥其生物增强作用。目前的研究提供了一种探索性策略,克服 BCS IV 药物的溶解度/溶出度差、渗透性差以及细胞色素 P450 同工酶的代谢,以提高其口服生物利用度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号