当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Engineering E. coli for magnetic control and the spatial localization of functions.
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-09-14 , DOI: 10.1021/acssynbio.0c00286 Mary Aubry 1 , Wei-An Wang 1, 2 , Yohan Guyodo 3 , Eugénia Delacou 1 , Jean-Michel Guigner 2 , Olivier Espeli 4 , Alice Lebreton 5, 6 , François Guyot 2, 7 , Zoher Gueroui 1
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2020-09-14 , DOI: 10.1021/acssynbio.0c00286 Mary Aubry 1 , Wei-An Wang 1, 2 , Yohan Guyodo 3 , Eugénia Delacou 1 , Jean-Michel Guigner 2 , Olivier Espeli 4 , Alice Lebreton 5, 6 , François Guyot 2, 7 , Zoher Gueroui 1
Affiliation
|
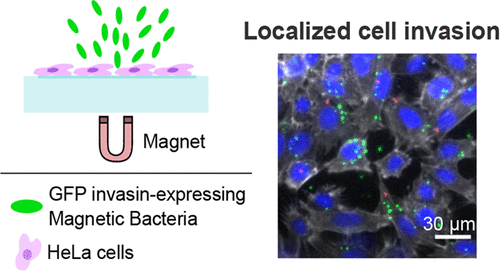
The fast-developing field of synthetic biology enables broad applications of programmed microorganisms including the development of whole-cell biosensors, delivery vehicles for therapeutics, or diagnostic agents. However, the lack of spatial control required for localizing microbial functions could limit their use and induce their dilution leading to ineffective action or dissemination. To overcome this limitation, the integration of magnetic properties into living systems enables a contact-less and orthogonal method for spatiotemporal control. Here, we generated a magnetic-sensing Escherichia coli by driving the formation of iron-rich bodies into bacteria. We found that these bacteria could be spatially controlled by magnetic forces and sustained cell growth and division, by transmitting asymmetrically their magnetic properties to one daughter cell. We combined the spatial control of bacteria with genetically encoded-adhesion properties to achieve the magnetic capture of specific target bacteria as well as the spatial modulation of human cell invasions.
中文翻译:

用于磁性控制和功能空间定位的工程大肠杆菌。
快速发展的合成生物学领域使程序化微生物的广泛应用成为可能,包括开发全细胞生物传感器、用于治疗的载体或诊断剂。然而,定位微生物功能所需的空间控制的缺乏可能会限制它们的使用并导致它们的稀释,从而导致无效的行动或传播。为了克服这一限制,将磁性集成到生命系统中可以实现一种非接触和正交的时空控制方法。在这里,我们生成了磁感应大肠杆菌通过推动富含铁的身体形成细菌。我们发现这些细菌可以通过磁力和持续的细胞生长和分裂在空间上控制,通过将它们的磁性不对称地传递给一个子细胞。我们将细菌的空间控制与遗传编码的粘附特性相结合,以实现对特定目标细菌的磁捕获以及人类细胞入侵的空间调节。
更新日期:2020-11-21
中文翻译:

用于磁性控制和功能空间定位的工程大肠杆菌。
快速发展的合成生物学领域使程序化微生物的广泛应用成为可能,包括开发全细胞生物传感器、用于治疗的载体或诊断剂。然而,定位微生物功能所需的空间控制的缺乏可能会限制它们的使用并导致它们的稀释,从而导致无效的行动或传播。为了克服这一限制,将磁性集成到生命系统中可以实现一种非接触和正交的时空控制方法。在这里,我们生成了磁感应大肠杆菌通过推动富含铁的身体形成细菌。我们发现这些细菌可以通过磁力和持续的细胞生长和分裂在空间上控制,通过将它们的磁性不对称地传递给一个子细胞。我们将细菌的空间控制与遗传编码的粘附特性相结合,以实现对特定目标细菌的磁捕获以及人类细胞入侵的空间调节。



























 京公网安备 11010802027423号
京公网安备 11010802027423号