当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereocomplementary and Parallel Syntheses of Multi-substituted (E)-, (Z)-Stereodefined α,β-Unsaturated Esters: Application to Drug Syntheses.
The Chemical Record ( IF 6.6 ) Pub Date : 2020-09-15 , DOI: 10.1002/tcr.202000076 Yuichiro Ashida 1 , Yoo Tanabe 1
The Chemical Record ( IF 6.6 ) Pub Date : 2020-09-15 , DOI: 10.1002/tcr.202000076 Yuichiro Ashida 1 , Yoo Tanabe 1
Affiliation
|
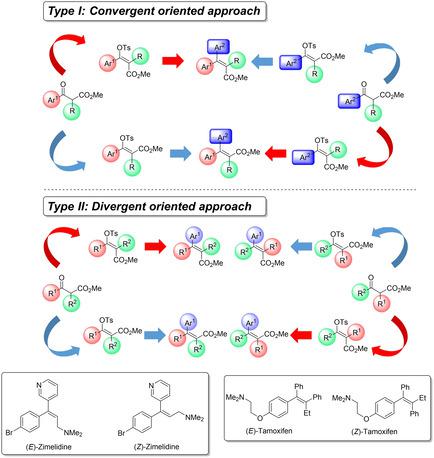
Ubiquitous α,β‐unsaturated esters are well recognized as key structural olefin scaffolds in organic chemistry. (E)‐ and (Z)‐steroselectivity is the most critical issue in their synthesis, however, (E)‐ and (Z)‐ stereocomplementary synthetic methods remain quite limited. The present account discloses general (E)‐, (Z)‐stereocomplementary syntheses of a variety of α,β‐unsaturated esters from highly accessible (E)‐, (Z)‐stereodefined enol tosylates derived from β‐ketoesters and α‐formyl esters. Step 1 toward the stereocomplementary preparation of (E)‐, (Z)‐stereodefined enol tosylates is implemented by using inexpensive reagents under mild reaction conditions. Step 2 toward the highly stereoretentive synthesis of (E)‐ and (Z)‐stereodefined α,β‐unsaturated esters involves Suzuki‐Miyaura, Negishi, Sonogashira, Iron‐catalyzed, Mizoroki‐Heck, and Buchwald‐Hartwig cross‐coupling reactions. Notably, this strategy was successfully applied for parallel drug syntheses of (E)‐ and (Z)‐zimelidine, (E)‐ and (Z)‐tamoxifen, and Merck's cyclopropane pharmacophore. Representative successful utilizations by other groups are also introduced.
中文翻译:

多取代的(E)-,(Z)-立体定义的α,β-不饱和酯的立体互补和平行合成:在药物合成中的应用。
普遍存在的α,β-不饱和酯是有机化学中关键的结构烯烃支架。(E)和(Z)立体选择性是它们合成中最关键的问题,但是(E)和(Z)立体互补合成方法仍然十分有限。本帐户公开了从β-酮酸酯和α-甲酰基衍生的高度可及的(E)-,(Z)-立体定义的烯醇甲苯磺酸酯中各种α,β-不饱和酯的一般(E)-,(Z)-立体互补合成酯。迈向(E)‐,(Z在温和的反应条件下,使用廉价的试剂可实现立体定义的烯醇甲苯磺酸盐。高度立体保持性合成(E)和(Z)-立体定义的α,β-不饱和酯的步骤2涉及Suzuki-Miyaura,Negishi,Sonogashira,铁催化,Mizoroki-Heck和Buchwald-Hartwig交叉偶联反应。值得注意的是,该策略已成功应用于(E)-和(Z)-齐美替丁,(E)-和(Z)-他莫昔芬以及默克的环丙烷药效团的平行药物合成。还介绍了其他小组的代表性成功利用方法。
更新日期:2020-09-15
中文翻译:

多取代的(E)-,(Z)-立体定义的α,β-不饱和酯的立体互补和平行合成:在药物合成中的应用。
普遍存在的α,β-不饱和酯是有机化学中关键的结构烯烃支架。(E)和(Z)立体选择性是它们合成中最关键的问题,但是(E)和(Z)立体互补合成方法仍然十分有限。本帐户公开了从β-酮酸酯和α-甲酰基衍生的高度可及的(E)-,(Z)-立体定义的烯醇甲苯磺酸酯中各种α,β-不饱和酯的一般(E)-,(Z)-立体互补合成酯。迈向(E)‐,(Z在温和的反应条件下,使用廉价的试剂可实现立体定义的烯醇甲苯磺酸盐。高度立体保持性合成(E)和(Z)-立体定义的α,β-不饱和酯的步骤2涉及Suzuki-Miyaura,Negishi,Sonogashira,铁催化,Mizoroki-Heck和Buchwald-Hartwig交叉偶联反应。值得注意的是,该策略已成功应用于(E)-和(Z)-齐美替丁,(E)-和(Z)-他莫昔芬以及默克的环丙烷药效团的平行药物合成。还介绍了其他小组的代表性成功利用方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号