当前位置:
X-MOL 学术
›
STEM CELLS
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Generation of hypoimmunogenic human pluripotent stem cells via expression of membrane-bound and secreted β2m-HLA-G fusion proteins
STEM CELLS ( IF 4.0 ) Pub Date : 2020-09-15 , DOI: 10.1002/stem.3269 Lei Shi 1, 2 , Wenjing Li 1 , Yang Liu 2, 3 , Zhenyu Chen 2, 3 , Yi Hui 2, 3 , Pengcheng Hao 2, 3 , Xiangjie Xu 2, 3 , Shuwei Zhang 2, 3 , Hexi Feng 2, 3 , Bowen Zhang 2, 3 , Shanshan Zhou 2, 3 , Nan Li 2, 3 , Lei Xiao 1, 4 , Ling Liu 2, 3, 5, 6 , Lin Ma 2, 3, 5, 6 , Xiaoqing Zhang 2, 3, 5, 6, 7, 8
STEM CELLS ( IF 4.0 ) Pub Date : 2020-09-15 , DOI: 10.1002/stem.3269 Lei Shi 1, 2 , Wenjing Li 1 , Yang Liu 2, 3 , Zhenyu Chen 2, 3 , Yi Hui 2, 3 , Pengcheng Hao 2, 3 , Xiangjie Xu 2, 3 , Shuwei Zhang 2, 3 , Hexi Feng 2, 3 , Bowen Zhang 2, 3 , Shanshan Zhou 2, 3 , Nan Li 2, 3 , Lei Xiao 1, 4 , Ling Liu 2, 3, 5, 6 , Lin Ma 2, 3, 5, 6 , Xiaoqing Zhang 2, 3, 5, 6, 7, 8
Affiliation
|
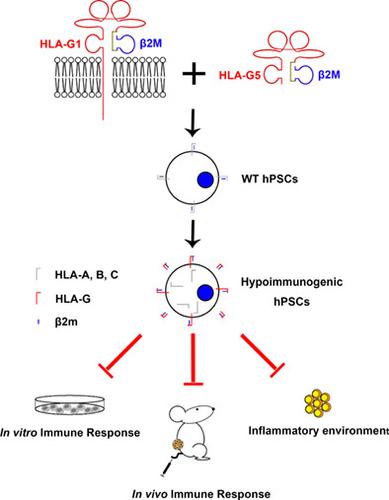
Allogeneic immune rejection is a major barrier for the application of human pluripotent stem cells (hPSCs) in regenerative medicine. A broad spectrum of immune cells, including T cells, natural killer (NK) cells, and antigen‐presenting cells, which either cause direct cell killing or constitute an immunogenic environment, are involved in allograft immune rejection. A strategy to protect donor cells from cytotoxicity while decreasing the secretion of inflammatory cytokines of lymphocytes is still lacking. Here, we engineered hPSCs with no surface expression of classical human leukocyte antigen (HLA) class I proteins via beta‐2 microglobulin (B2M) knockout or biallelic knockin of HLA‐G1 within the frame of endogenous B2M loci. Elimination of the surface expression of HLA class I proteins protected the engineered hPSCs from cytotoxicity mediated by T cells. However, this lack of surface expression also resulted in missing‐self response and NK cell activation, which were largely compromised by expression of β2m‐HLA‐G1 fusion proteins. We also proved that the engineered β2m‐HLA‐G5 fusion proteins were soluble, secretable, and capable of safeguarding low immunogenic environments by lowering inflammatory cytokines secretion in allografts. Our current study reveals a novel strategy that may offer unique advantages to construct hypoimmunogenic hPSCs via the expression of membrane‐bound and secreted β2m‐HLA‐G fusion proteins. These engineered hPSCs are expected to serve as an unlimited cell source for generating universally compatible “off‐the‐shelf” cell grafts in the future.
中文翻译:

通过表达膜结合和分泌的 β2m-HLA-G 融合蛋白产生低免疫原性人多能干细胞
同种异体免疫排斥是人类多能干细胞 (hPSC) 在再生医学中应用的主要障碍。广泛的免疫细胞,包括 T 细胞、自然杀伤 (NK) 细胞和抗原呈递细胞,它们要么导致直接细胞杀伤,要么构成免疫原性环境,参与同种异体移植免疫排斥反应。仍然缺乏保护供体细胞免受细胞毒性同时减少淋巴细胞炎性细胞因子分泌的策略。在这里,我们通过β2微球蛋白(B2M)敲除或内源性B2M位点框架内HLA-G1的双等位基因敲除,设计了没有经典人类白细胞抗原(HLA)I类蛋白表面表达的hPSC。消除 HLA I 类蛋白的表面表达保护工程化 hPSC 免受 T 细胞介导的细胞毒性。然而,这种表面表达的缺乏也导致了自我反应的缺失和 NK 细胞的激活,这在很大程度上受到了 β2m-HLA-G1 融合蛋白的表达的影响。我们还证明了工程化的 β2m-HLA-G5 融合蛋白是可溶的、可分泌的,并且能够通过降低同种异体移植物中的炎性细胞因子分泌来保护低免疫原性环境。我们目前的研究揭示了一种新策略,该策略可能为通过表达膜结合和分泌的 β2m-HLA-G 融合蛋白构建低免疫原性 hPSC 提供独特的优势。这些经过工程改造的 hPSC 有望在未来作为一种无限的细胞来源,用于生成普遍兼容的“现成”细胞移植物。
更新日期:2020-09-15
中文翻译:

通过表达膜结合和分泌的 β2m-HLA-G 融合蛋白产生低免疫原性人多能干细胞
同种异体免疫排斥是人类多能干细胞 (hPSC) 在再生医学中应用的主要障碍。广泛的免疫细胞,包括 T 细胞、自然杀伤 (NK) 细胞和抗原呈递细胞,它们要么导致直接细胞杀伤,要么构成免疫原性环境,参与同种异体移植免疫排斥反应。仍然缺乏保护供体细胞免受细胞毒性同时减少淋巴细胞炎性细胞因子分泌的策略。在这里,我们通过β2微球蛋白(B2M)敲除或内源性B2M位点框架内HLA-G1的双等位基因敲除,设计了没有经典人类白细胞抗原(HLA)I类蛋白表面表达的hPSC。消除 HLA I 类蛋白的表面表达保护工程化 hPSC 免受 T 细胞介导的细胞毒性。然而,这种表面表达的缺乏也导致了自我反应的缺失和 NK 细胞的激活,这在很大程度上受到了 β2m-HLA-G1 融合蛋白的表达的影响。我们还证明了工程化的 β2m-HLA-G5 融合蛋白是可溶的、可分泌的,并且能够通过降低同种异体移植物中的炎性细胞因子分泌来保护低免疫原性环境。我们目前的研究揭示了一种新策略,该策略可能为通过表达膜结合和分泌的 β2m-HLA-G 融合蛋白构建低免疫原性 hPSC 提供独特的优势。这些经过工程改造的 hPSC 有望在未来作为一种无限的细胞来源,用于生成普遍兼容的“现成”细胞移植物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号