当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Synthesis and Biological Evaluation of New Thieno[2,3‐g]indolizine Derivatives
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-09-15 , DOI: 10.1002/slct.202002684 Anna D. Zinoveva 1 , Tatiana N. Borisova 1 , Polina A. Politova 1 , Alexander A. Titov 1 , Alexey V. Varlamov 1 , Leonid G. Voskressensky 1 , Van Tuyen Nguyen 2 , Tuan Anh Le 3
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-09-15 , DOI: 10.1002/slct.202002684 Anna D. Zinoveva 1 , Tatiana N. Borisova 1 , Polina A. Politova 1 , Alexander A. Titov 1 , Alexey V. Varlamov 1 , Leonid G. Voskressensky 1 , Van Tuyen Nguyen 2 , Tuan Anh Le 3
Affiliation
|
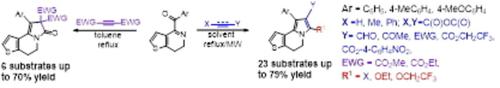
An effective approach to the synthesis of thieno[2,3‐g]indolizine derivatives has been developed. A wide variety of reagents such as unsaturated aldehydes, methylvinylketone, esters of acrylic acid, maleic anhydride, methyl propiolate, dimethyl and diethyl acetylenedicarboxylates in the construction of functionally diverse rarely encountered tricyclic system has been used. A high number of structurally various thieno[2,3‐g]indolizines has been obtained in domino reactions with medium and high yields, the influence of solvents on the course of transformations has been studied, and the presence of moderate cytotoxic activity for a number of compounds has been established.
中文翻译:

新的噻吩并[2,3-g]吲哚嗪衍生物的简便合成及生物学评价
已经开发了一种合成噻吩并[2,3- g ]吲哚嗪衍生物的有效方法。在功能上很少遇到的三环体系的构建中,已经使用了各种各样的试剂,例如不饱和醛,甲基乙烯基酮,丙烯酸的酯,马来酸酐,丙炔酸甲酯,乙炔基二羧酸二甲酯和乙二酸二乙酯。在多米诺骨牌反应中以中等和高收率获得了大量结构上不同的噻吩并[2,3-g]吲哚并酮,研究了溶剂对转化过程的影响,并且存在中等程度的细胞毒活性已经建立化合物。
更新日期:2020-09-15
中文翻译:

新的噻吩并[2,3-g]吲哚嗪衍生物的简便合成及生物学评价
已经开发了一种合成噻吩并[2,3- g ]吲哚嗪衍生物的有效方法。在功能上很少遇到的三环体系的构建中,已经使用了各种各样的试剂,例如不饱和醛,甲基乙烯基酮,丙烯酸的酯,马来酸酐,丙炔酸甲酯,乙炔基二羧酸二甲酯和乙二酸二乙酯。在多米诺骨牌反应中以中等和高收率获得了大量结构上不同的噻吩并[2,3-g]吲哚并酮,研究了溶剂对转化过程的影响,并且存在中等程度的细胞毒活性已经建立化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号