Water Research ( IF 11.4 ) Pub Date : 2020-09-15 , DOI: 10.1016/j.watres.2020.116412 Shun Saito 1 , Yoshihiko Matsui 2 , Yasuhiko Yamamoto 3 , Shuhei Matsushita 4 , Satoru Mima 3 , Nobutaka Shirasaki 2 , Taku Matsushita 2
|
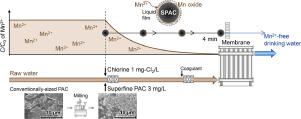
Here, we examined the removal of soluble divalent manganese (Mn(II)) by combination treatment with superfine powdered activated carbon (SPAC) and free chlorine in a membrane filtration pilot plant and batch experiments. Removal rates >95% were obtained with 3 mg/L SPAC, 1 mg/L chlorine, and a contact time of 4 min, meeting practical performance standards. Mn(II) was found to be oxidized and precipitated on the surface of the activated carbon particles by chlorine. The Mn(II) removal rate was fitted to pseudo-first-order reaction kinetics, and the rate coefficient changed in inverse proportion to as-is particle size, but not to true particle size. The rate coefficient was independent of both Mn(II) concentration, except at high Mn(II) concentration, and the chlorine concentrations tested. The rate-determining step of Mn(II) removal was confirmed to be external-film mass transfer, not chemical oxidation. Activated carbon was found to have a catalytic effect on the oxidation of Mn(II), but the effect was minimal for conventionally sized activated carbon. However, Mn(II) removal at feasible rates for practical application can be expected when the activated carbon particle diameter is reduced to several micrometers. Activated carbon with a particle size of around 1–2 μm may be the most appropriate for Mn(II) removal because particles below this size were aggregated, resulting in reduced removal efficiency.
中文翻译:

超微粉活性炭存在下氯气氧化去除可溶性二价锰离子
在这里,我们研究了在膜过滤中试和批量实验中通过超细粉状活性炭 (SPAC) 和游离氯的组合处理去除可溶性二价锰 (Mn(II))。使用 3 mg/L SPAC、1 mg/L 氯和 4 分钟接触时间获得 >95% 的去除率,满足实际性能标准。发现Mn(II)被氯氧化并沉淀在活性炭颗粒表面。Mn(II) 去除率符合准一级反应动力学,并且速率系数与原样粒径成反比,但与真实粒径成反比。速率系数与 Mn(II) 浓度无关,除了在高 Mn(II) 浓度下,以及测试的氯浓度。Mn(II) 去除的速度决定步骤被证实是外膜传质,而不是化学氧化。发现活性炭对 Mn(II) 的氧化具有催化作用,但对于常规尺寸的活性炭,该作用最小。然而,当活性炭粒径减小到几微米时,可以预期在实际应用中以可行的速率去除 Mn(II)。粒径在 1-2 μm 左右的活性炭可能最适合去除 Mn(II),因为低于此尺寸的颗粒会聚集,导致去除效率降低。当活性炭粒径减小到几微米时,可以预期在实际应用中以可行的速率去除 Mn(II)。粒径在 1-2 μm 左右的活性炭可能最适合去除 Mn(II),因为低于此尺寸的颗粒会聚集,导致去除效率降低。当活性炭粒径减小到几微米时,可以预期在实际应用中以可行的速率去除 Mn(II)。粒径在 1-2 μm 左右的活性炭可能最适合去除 Mn(II),因为低于此尺寸的颗粒会聚集,导致去除效率降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号