Structure ( IF 4.4 ) Pub Date : 2020-09-15 , DOI: 10.1016/j.str.2020.08.011 Steven E Cohen 1 , Edward J Brignole 2 , Elizabeth C Wittenborn 1 , Mehmet Can 3 , Samuel Thompson 1 , Stephen W Ragsdale 3 , Catherine L Drennan 4
|
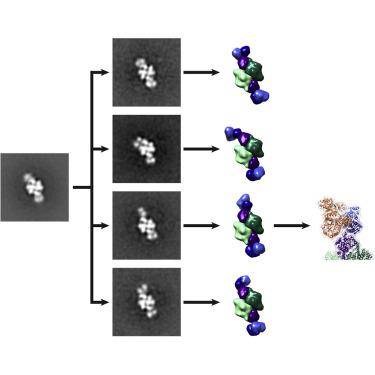
The Ni-Fe-S-containing A-cluster of acetyl-coenzyme A (CoA) synthase (ACS) assembles acetyl-CoA from carbon monoxide (CO), a methyl group (CH3+), and CoA. To accomplish this feat, ACS must bind CoA and interact with two other proteins that contribute the CO and CH3+, respectively: CO dehydrogenase (CODH) and corrinoid Fe-S protein (CFeSP). Previous structural data show that, in the model acetogen Moorella thermoacetica, domain 1 of ACS binds to CODH such that a 70-Å-long internal channel is created that allows CO to travel from CODH to the A-cluster. The A-cluster is largely buried and is inaccessible to CFeSP for methylation. Here we use electron microscopy to capture multiple snapshots of ACS that reveal previously uncharacterized domain motion, forming extended and hyperextended structural states. In these structural states, the A-cluster is accessible for methylation by CFeSP.
中文翻译:

负染色电子显微镜揭示了 Ni-Fe-S 依赖性一氧化碳脱氢酶/乙酰辅酶 A 合成酶中显着的结构重排。
乙酰辅酶 A (CoA) 合酶 (ACS) 的含 Ni-Fe-S 的 A 簇由一氧化碳 (CO)、甲基 (CH 3 + ) 和 CoA 组装乙酰辅酶 A。为了实现这一壮举,ACS 必须结合 CoA 并与另外两种分别提供 CO 和 CH 3 +的蛋白质相互作用:CO 脱氢酶 (CODH) 和类咕啉 Fe-S 蛋白 (CFeSP)。先前的结构数据表明,在产乙酸莫雷拉热乙酸模型中,ACS 的结构域 1 与 CODH 结合,从而创建一个 70 Å 长的内部通道,允许 CO 从 CODH 传输到 A 簇。 A 簇大部分被掩埋,CFeSP 无法进行甲基化。在这里,我们使用电子显微镜捕获 ACS 的多个快照,这些快照揭示了以前未表征的域运动,形成了扩展和超扩展的结构状态。在这些结构状态下,A 簇可被 CFeSP 甲基化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号