Cell Reports ( IF 7.5 ) Pub Date : 2020-09-15 , DOI: 10.1016/j.celrep.2020.108133 Ruiliang Zhao 1 , Yang Yang 1 , Fan Zheng 1 , Zhifeng Zeng 1 , Wenqian Feng 1 , Xuexia Jin 1 , Jiayi Wang 1 , Ke Yang 1 , Yun Xiang Liang 1 , Qunxin She 2 , Wenyuan Han 1
|
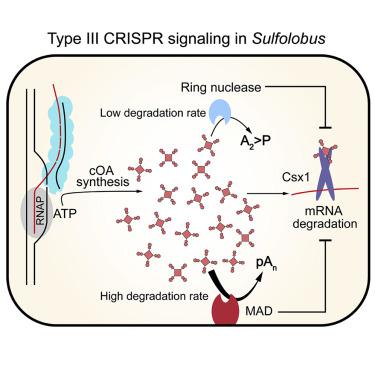
Type III CRISPR-Cas systems initiate an intracellular signaling pathway to confer immunity. The signaling pathway includes synthesis of cyclic oligo-adenylate (cOA) and activation of the RNase activity of type III accessory ribonuclease Csm6/Csx1 by cOA. After the immune response, cOA should be cleared on time to avoid constant cellular RNA degradation. In this study, we find a metal-dependent cOA degradation activity in Sulfolobus islandicus. The activity is associated with the cell membrane and able to accelerate cOA clearance at a high cOA level. Further, we show that a metal-dependent and membrane-associated DHH-DHHA1 family nuclease (MAD) rapidly cleaves cOA and deactivates Csx1 ribonuclease. The cOA degradation efficiency of MAD is much higher than the cellular ring nuclease. However, the subcellular organization may prevent it from degrading nascent cOA. Together, the data suggest that MAD acts as the second cOA degrader after the ring nuclease to remove diffused redundant cOA.
中文翻译:

膜相关的DHH-DHHA1核酸酶降解III型CRISPR第二信使。
III型CRISPR-Cas系统启动细胞内信号传导途径以赋予免疫力。信号传导途径包括环状寡聚腺苷酸(cOA)的合成和cOA激活III型辅助核糖核酸酶Csm6 / Csx1的RNase活性。免疫反应后,应及时清除cOA,以避免持续的细胞RNA降解。在这项研究中,我们发现了Sulfolobus islandicus中的金属依赖性cOA降解活性。活性与细胞膜有关,并能在高cOA水平下加速cOA清除。此外,我们显示,金属依赖性和膜相关的DHH-DHHA1家族核酸酶(MAD)迅速裂解cOA并失活Csx1核糖核酸酶。MAD的cOA降解效率比细胞环核酸酶高得多。但是,亚细胞组织可能阻止其降解新生的cOA。总之,数据表明,MAD充当环核酸酶之后的第二个cOA降解剂,以去除扩散的冗余cOA。











































 京公网安备 11010802027423号
京公网安备 11010802027423号