Cell ( IF 45.5 ) Pub Date : 2020-09-15 , DOI: 10.1016/j.cell.2020.08.023 Alexandra Serris 1 , Robert Stass 2 , Eduardo A Bignon 3 , Nicolás A Muena 4 , Jean-Claude Manuguerra 5 , Rohit K Jangra 6 , Sai Li 7 , Kartik Chandran 6 , Nicole D Tischler 3 , Juha T Huiskonen 8 , Felix A Rey 1 , Pablo Guardado-Calvo 1
|
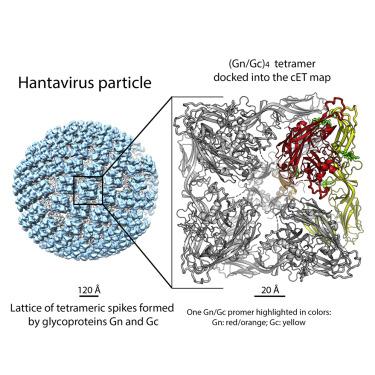
Hantaviruses are rodent-borne viruses causing serious zoonotic outbreaks worldwide for which no treatment is available. Hantavirus particles are pleomorphic and display a characteristic square surface lattice. The envelope glycoproteins Gn and Gc form heterodimers that further assemble into tetrameric spikes, the lattice building blocks. The glycoproteins, which are the sole targets of neutralizing antibodies, drive virus entry via receptor-mediated endocytosis and endosomal membrane fusion. Here we describe the high-resolution X-ray structures of the heterodimer of Gc and the Gn head and of the homotetrameric Gn base. Docking them into an 11.4-Å-resolution cryoelectron tomography map of the hantavirus surface accounted for the complete extramembrane portion of the viral glycoprotein shell and allowed a detailed description of the surface organization of these pleomorphic virions. Our results, which further revealed a built-in mechanism controlling Gc membrane insertion for fusion, pave the way for immunogen design to protect against pathogenic hantaviruses.
中文翻译:

汉坦病毒表面糖蛋白点阵及其融合控制机制。
汉坦病毒是啮齿动物传播的病毒,在世界范围内引起严重的人畜共患病暴发,目前尚无治疗方法。汉坦病毒颗粒是多形性的,并显示出特征性的方形表面晶格。包膜糖蛋白 Gn 和 Gc 形成异二聚体,进一步组装成四聚体尖峰,即晶格构建块。糖蛋白是中和抗体的唯一目标,通过受体介导的内吞作用和内体膜融合驱动病毒进入。在这里,我们描述了 Gc 和 Gn 头的异二聚体以及同四聚体 Gn 碱基的高分辨率 X 射线结构。将它们对接成 11。汉坦病毒表面的 4 Å 分辨率冷冻电子断层扫描图解释了病毒糖蛋白外壳的完整膜外部分,并允许详细描述这些多形病毒粒子的表面组织。我们的结果进一步揭示了控制 Gc 膜插入融合的内置机制,为免疫原设计以防止致病性汉坦病毒感染铺平了道路。









































 京公网安备 11010802027423号
京公网安备 11010802027423号