Cell ( IF 45.5 ) Pub Date : 2020-09-14 , DOI: 10.1016/j.cell.2020.09.033 Thomas Mandel Clausen 1 , Daniel R Sandoval 2 , Charlotte B Spliid 1 , Jessica Pihl 1 , Hailee R Perrett 3 , Chelsea D Painter 4 , Anoop Narayanan 5 , Sydney A Majowicz 5 , Elizabeth M Kwong 6 , Rachael N McVicar 6 , Bryan E Thacker 7 , Charles A Glass 7 , Zhang Yang 8 , Jonathan L Torres 3 , Gregory J Golden 4 , Phillip L Bartels 9 , Ryan N Porell 10 , Aaron F Garretson 11 , Logan Laubach 10 , Jared Feldman 12 , Xin Yin 13 , Yuan Pu 13 , Blake M Hauser 12 , Timothy M Caradonna 12 , Benjamin P Kellman 14 , Cameron Martino 15 , Philip L S M Gordts 16 , Sumit K Chanda 13 , Aaron G Schmidt 17 , Kamil Godula 18 , Sandra L Leibel 19 , Joyce Jose 5 , Kevin D Corbett 2 , Andrew B Ward 3 , Aaron F Carlin 11 , Jeffrey D Esko 9
|
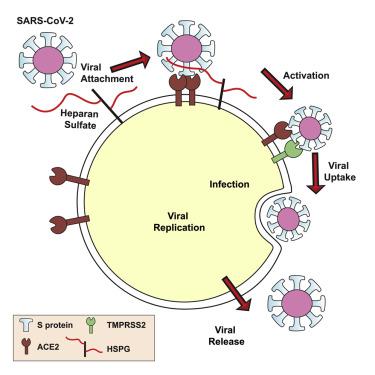
We show that SARS-CoV-2 spike protein interacts with both cellular heparan sulfate and angiotensin-converting enzyme 2 (ACE2) through its receptor-binding domain (RBD). Docking studies suggest a heparin/heparan sulfate-binding site adjacent to the ACE2-binding site. Both ACE2 and heparin can bind independently to spike protein in vitro, and a ternary complex can be generated using heparin as a scaffold. Electron micrographs of spike protein suggests that heparin enhances the open conformation of the RBD that binds ACE2. On cells, spike protein binding depends on both heparan sulfate and ACE2. Unfractionated heparin, non-anticoagulant heparin, heparin lyases, and lung heparan sulfate potently block spike protein binding and/or infection by pseudotyped virus and authentic SARS-CoV-2 virus. We suggest a model in which viral attachment and infection involves heparan sulfate-dependent enhancement of binding to ACE2. Manipulation of heparan sulfate or inhibition of viral adhesion by exogenous heparin presents new therapeutic opportunities.
中文翻译:

SARS-CoV-2 感染依赖于细胞硫酸乙酰肝素和 ACE2
我们发现 SARS-CoV-2 刺突蛋白通过其受体结合域 (RBD) 与细胞硫酸乙酰肝素和血管紧张素转换酶 2 (ACE2) 相互作用。对接研究表明肝素/硫酸乙酰肝素结合位点与 ACE2 结合位点相邻。 ACE2和肝素均可在体外独立地与刺突蛋白结合,并且可以使用肝素作为支架生成三元复合物。刺突蛋白的电子显微照片表明,肝素增强了结合 ACE2 的 RBD 的开放构象。在细胞上,刺突蛋白的结合取决于硫酸乙酰肝素和 ACE2。普通肝素、非抗凝肝素、肝素裂解酶和肺硫酸乙酰肝素可有效阻断假型病毒和真正的 SARS-CoV-2 病毒的刺突蛋白结合和/或感染。我们提出了一种模型,其中病毒附着和感染涉及硫酸乙酰肝素依赖性增强与 ACE2 的结合。操纵硫酸乙酰肝素或通过外源性肝素抑制病毒粘附提供了新的治疗机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号