当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhenium-catalyzed alkylarylation of alkenes with PhI(O2CR)2via decarboxylation to access indolinones and dihydroquinolinones
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-14 , DOI: 10.1039/d0qo00953a Lei Zhang 1, 2, 3, 4, 5 , Yin Wang 6, 7, 8, 9, 10 , Yunhui Yang 6, 7, 8, 9, 10 , Ping Zhang 1, 2, 3, 4, 5 , Congyang Wang 6, 7, 8, 9, 10
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-09-14 , DOI: 10.1039/d0qo00953a Lei Zhang 1, 2, 3, 4, 5 , Yin Wang 6, 7, 8, 9, 10 , Yunhui Yang 6, 7, 8, 9, 10 , Ping Zhang 1, 2, 3, 4, 5 , Congyang Wang 6, 7, 8, 9, 10
Affiliation
|
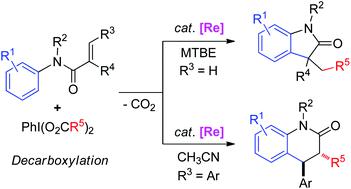
Rhenium-catalyzed alkylarylation of alkenes with hypervalent iodine(III) reagents (HIRs) via decarboxylation to access various 3,3-disubstituted indolinones and trans-3,4-dihydroquinolinones is described. These transformations feature wide substrate scopes, good functional-group tolerance, and divergent regioselectivity. Mechanistic studies revealed a cascade sequence of decarboxylation/intermolecular radical alkylation/intramolecular radical arylation/oxidative re-aromatization.
中文翻译:

Ph通过羰基催化的PhI(O2CR)2烯烃与烯烃的烷基芳基化反应,以得到吲哚啉酮和二氢喹啉酮
描述了通过高羧基碘(III)试剂(HIR)进行的催化烯烃的烷基芳基化反应,从而获得各种3,3-二取代的吲哚啉酮和反式-3,4-二氢喹啉酮。这些转变具有广泛的底物范围,良好的官能团耐受性和不同的区域选择性。机理研究揭示了脱羧/分子间自由基烷基化/分子内自由基芳基化/氧化再芳构化的级联序列。
更新日期:2020-10-13
中文翻译:

Ph通过羰基催化的PhI(O2CR)2烯烃与烯烃的烷基芳基化反应,以得到吲哚啉酮和二氢喹啉酮
描述了通过高羧基碘(III)试剂(HIR)进行的催化烯烃的烷基芳基化反应,从而获得各种3,3-二取代的吲哚啉酮和反式-3,4-二氢喹啉酮。这些转变具有广泛的底物范围,良好的官能团耐受性和不同的区域选择性。机理研究揭示了脱羧/分子间自由基烷基化/分子内自由基芳基化/氧化再芳构化的级联序列。











































 京公网安备 11010802027423号
京公网安备 11010802027423号