当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Equilibria in Binary Mixtures of Water with Triglyme and Tetraglyme: Experimental Determination and Thermodynamic Modeling
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-14 , DOI: 10.1021/acs.jced.0c00600 Ondřej Kolín 1 , Vladimír Dohnal 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-09-14 , DOI: 10.1021/acs.jced.0c00600 Ondřej Kolín 1 , Vladimír Dohnal 1
Affiliation
|
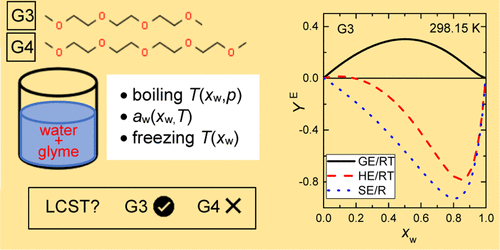
Because of their many favorable properties and specifically balanced amphiphilic nature, glymes (oligomeric ethylene glycol diethers) are of great technological and theoretical interest. This study focuses on the phase equilibria and energetics of aqueous solutions of two important members of the glyme series, triglyme (triethylene glycol dimethyl ether) and tetraglyme (tetraethylene glycol dimethyl ether). For these systems, we carried out accurate measurements of water activity at two temperatures (298.15 and 313.15 or 318.15 K) in the whole composition range, boiling temperatures at three pressures (50, 70, and 90 kPa), and freezing temperatures in the water-rich region. The melting temperature and melting enthalpy of the neat glymes were also determined. We correlated our water activity data simultaneously with some related thermal data from the literature using an extended SSF-type excess Gibbs energy (GE) model. The established model descriptions provided not only particularly good fit of the underlying data but, as proven by due comparisons to other results from both the literature and this work, showed a superior performance, extrapolating very well to both higher and lower temperatures. The high-fidelity global modeling also enabled us to present a clear picture of the energetics of the two aqueous glymes. At near-ambient temperatures, both systems exhibit non-monotonous activity coefficient courses with composition, large exothermic effects, and remarkably deep drops of the entropy accompanying the mixing, their positive GE being entropy-driven. On increasing the temperature, the former features gradually decline while GE keeps about the same magnitude and becomes enthalpy-driven. Although both systems show a great tendency to phase splitting above the normal boiling temperature of water, our model calculations predicted the lower critical solution temperature behavior only for the aqueous solution of triglyme. The observed pattern of the energetic behavior has been well elucidated in terms of competitive water–water and water–ether H-bonding.
中文翻译:

水与三甘醇二甲醚和四甘醇二甲醚的二元混合物中的相平衡:实验确定和热力学模型
由于甘醇二甲醚(低聚乙二醇二醚)具有许多有利的特性和特别平衡的两亲性质,因此在技术和理论上都具有极大的意义。这项研究的重点是甘醇二甲醚(三甘醇二甲醚二甲醚)和四甘醇二甲醚(四甘醇二甲醚)这两个甘醇二甲醚系列重要成员的水溶液的相平衡和高能。对于这些系统,我们在整个组成范围内的两个温度(298.15和313.15或318.15 K),三个压力(50、70和90 kPa)下的沸腾温度以及水中的冻结温度下,精确测量了水活度丰富的地区。还确定了纯胶体的熔融温度和熔融焓。G E)模型。既定的模型描述不仅提供了对基础数据的特别合适的拟合,而且通过与文献和这项工作的其他结果进行了适当的比较证明,该模型说明显示了优越的性能,可以很好地推断出较高和较低的温度。高保真全局建模还使我们能够清晰地显示两种水性甘醇二甲醚的能量。在接近环境温度的情况下,两个系统均具有非单调活度系数曲线,具有组成,较大的放热效应以及伴随混合而产生的熵的明显下降,其正G E受熵驱动。随着温度的升高,前者的特征逐渐降低,而G E保持相同的幅度,并成为焓驱动的。尽管两个系统都显示出在水的正常沸腾温度以上有很大的相分离趋势,但我们的模型计算预测,仅对于三甘醇二甲醚水溶液,临界溶液的温度行为较低。从竞争性的水—水和水—醚氢键的竞争中,已经很好地阐明了观察到的能量行为模式。
更新日期:2020-10-08
中文翻译:

水与三甘醇二甲醚和四甘醇二甲醚的二元混合物中的相平衡:实验确定和热力学模型
由于甘醇二甲醚(低聚乙二醇二醚)具有许多有利的特性和特别平衡的两亲性质,因此在技术和理论上都具有极大的意义。这项研究的重点是甘醇二甲醚(三甘醇二甲醚二甲醚)和四甘醇二甲醚(四甘醇二甲醚)这两个甘醇二甲醚系列重要成员的水溶液的相平衡和高能。对于这些系统,我们在整个组成范围内的两个温度(298.15和313.15或318.15 K),三个压力(50、70和90 kPa)下的沸腾温度以及水中的冻结温度下,精确测量了水活度丰富的地区。还确定了纯胶体的熔融温度和熔融焓。G E)模型。既定的模型描述不仅提供了对基础数据的特别合适的拟合,而且通过与文献和这项工作的其他结果进行了适当的比较证明,该模型说明显示了优越的性能,可以很好地推断出较高和较低的温度。高保真全局建模还使我们能够清晰地显示两种水性甘醇二甲醚的能量。在接近环境温度的情况下,两个系统均具有非单调活度系数曲线,具有组成,较大的放热效应以及伴随混合而产生的熵的明显下降,其正G E受熵驱动。随着温度的升高,前者的特征逐渐降低,而G E保持相同的幅度,并成为焓驱动的。尽管两个系统都显示出在水的正常沸腾温度以上有很大的相分离趋势,但我们的模型计算预测,仅对于三甘醇二甲醚水溶液,临界溶液的温度行为较低。从竞争性的水—水和水—醚氢键的竞争中,已经很好地阐明了观察到的能量行为模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号