当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of the Molecular Structure and Elemental Mercury Adsorption Mechanism of Biomass Char
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-09-14 , DOI: 10.1021/acs.energyfuels.0c02626 Li Jia 1 , Yue Yu 2 , Jin-rong Guo 1 , Shu-ning Qin 1 , Yan-lin Wang 1 , Xin Shen 1 , Bao-guo Fan 1 , Yan Jin 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-09-14 , DOI: 10.1021/acs.energyfuels.0c02626 Li Jia 1 , Yue Yu 2 , Jin-rong Guo 1 , Shu-ning Qin 1 , Yan-lin Wang 1 , Xin Shen 1 , Bao-guo Fan 1 , Yan Jin 1
Affiliation
|
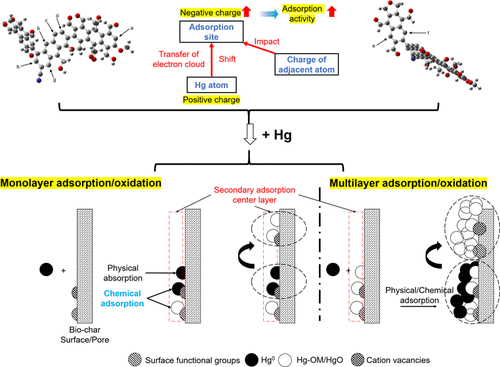
Based on a comprehensive study of the pyrolysis characteristics, pore structure, surface chemical functional groups, organic carbon framework structure, microcrystalline morphology, and lattice characteristics of walnut shell biochar for mercury adsorption, the structural characteristics of biochar were analyzed, and a model of the monomeric molecular structure of biochar was constructed. In addition, the density functional theory of quantum mechanics was introduced into the gas–solid adsorption reaction of elemental mercury with biochar. By quantitatively studying the adsorption energy, adsorption height, and Mulliken population number of the adsorption system, the adsorption mechanism of Hg0 by biochar was revealed to provide a theoretical basis for future mercury removal methods. The results showed that walnut shell biochar consisted mainly of C, H, O, and N. Aromatic carbon was the main component of the biochar molecular structure, while aliphatic carbon was linked to aromatic structural units. The molecular structure model was mainly composed of aromatic structures, including one methyl, four hydroxyl, and eight carbonyl groups. The molecular formula was C55H37NO14, and the molecular weight was 935. The adsorption of Hg0 by biochar was mainly via chemical adsorption, and the adsorbed Hg0 could stably exist on the biochar surface. The Hg0 adsorption process on the biochar surface mainly depended on the charge of the adsorption site. An adsorption site with a negative charge and with a high number of charges facilitated the adsorption of elemental mercury on the biochar surface. Moreover, the charge of the ortho atom adjacent to the adsorption site had a substantial influence on the adsorption activity of the adsorption site.
中文翻译:

生物质炭的分子结构和元素汞吸附机理的研究
在对核桃壳生物炭吸附汞的热解特性,孔结构,表面化学官能团,有机碳骨架结构,微晶形态和晶格特性进行综合研究的基础上,分析了生物炭的结构特征,并建立了模型。构建了生物炭的单体分子结构。另外,量子力学的密度泛函理论被引入到元素汞与生物炭的气固吸附反应中。通过定量研究吸附体系的吸附能,吸附高度和Mulliken数,得出Hg 0的吸附机理。生物炭的生物分解被揭示为将来的汞去除方法提供了理论基础。结果表明,核桃壳生物碳主要由碳,氢,氧和氮组成。芳香碳是生物碳分子结构的主要成分,而脂肪族碳则与芳香族结构单元相连。分子结构模型主要由芳族结构组成,包括一个甲基,四个羟基和八个羰基。分子式为C 55 H 37 NO 14,分子量为935。生物炭对Hg 0的吸附主要是化学吸附,被吸附的Hg 0可以稳定地存在于生物炭的表面。汞0生物炭表面的吸附过程主要取决于吸附位的电荷。带负电荷且带大量电荷的吸附位点促进了元素汞在生物炭表面的吸附。此外,与吸附位点相邻的邻原子的电荷对吸附位点的吸附活性具有实质性的影响。
更新日期:2020-10-16
中文翻译:

生物质炭的分子结构和元素汞吸附机理的研究
在对核桃壳生物炭吸附汞的热解特性,孔结构,表面化学官能团,有机碳骨架结构,微晶形态和晶格特性进行综合研究的基础上,分析了生物炭的结构特征,并建立了模型。构建了生物炭的单体分子结构。另外,量子力学的密度泛函理论被引入到元素汞与生物炭的气固吸附反应中。通过定量研究吸附体系的吸附能,吸附高度和Mulliken数,得出Hg 0的吸附机理。生物炭的生物分解被揭示为将来的汞去除方法提供了理论基础。结果表明,核桃壳生物碳主要由碳,氢,氧和氮组成。芳香碳是生物碳分子结构的主要成分,而脂肪族碳则与芳香族结构单元相连。分子结构模型主要由芳族结构组成,包括一个甲基,四个羟基和八个羰基。分子式为C 55 H 37 NO 14,分子量为935。生物炭对Hg 0的吸附主要是化学吸附,被吸附的Hg 0可以稳定地存在于生物炭的表面。汞0生物炭表面的吸附过程主要取决于吸附位的电荷。带负电荷且带大量电荷的吸附位点促进了元素汞在生物炭表面的吸附。此外,与吸附位点相邻的邻原子的电荷对吸附位点的吸附活性具有实质性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号