当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and toxicity of halogenated bisphenol monosubstituted‐ethers: Establishing a library for potential environmental transformation products of emerging contaminant
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-10-16 , DOI: 10.1002/cbdv.202000481 Rui Guo 1, 2 , Mengxi Cao 1 , Ming Hu 1 , Wenchao Deng 1 , Wenjuan Zhang 1 , Yangguang Gao 3 , Shihan Ye 4 , Weixiang Zhou 4 , Jianbo Shi 1, 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-10-16 , DOI: 10.1002/cbdv.202000481 Rui Guo 1, 2 , Mengxi Cao 1 , Ming Hu 1 , Wenchao Deng 1 , Wenjuan Zhang 1 , Yangguang Gao 3 , Shihan Ye 4 , Weixiang Zhou 4 , Jianbo Shi 1, 2
Affiliation
|
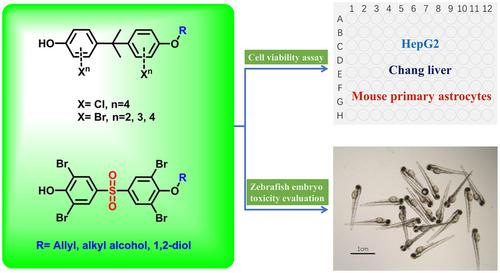
As an important branch of halogenated bisphenol compounds, the halogenated bisphenol monosubstituted‐ether compounds have received a lot of attention in environmental health science because of their toxicity and variability. In this study, a synthetic method for bisphenol monosubstituted‐ether byproduct libraries was developed. By using the versatile and efficient method, tetrachlorobisphenol A, tetrabromobisphenol A, and tetrabromobisphenol S monosubstituted alkyl‐ether compounds were accessed in 39–82 % yield. Subsequently, the cytotoxicity of 27 compounds were screened using three different cell lines (HepG2, mouse primary astrocytes and Chang liver cells). Compound 2,6‐dibromo‐4‐[3,5‐dibromo‐4‐(2‐hydroxyethoxy)benzene‐1‐sulfonyl]phenol was more toxic than other compounds in various cells, and the sensitivity of this compound to the normal hepatocytes and cancer cells was inconsistent. The compounds 2,6‐dichloro‐4‐(2‐{3,5‐dichloro‐4‐[(prop‐2‐en‐1‐yl)oxy]phenyl}propan‐2‐yl)phenol and 2,6‐dibromo‐4‐(2‐{3,5‐dibromo‐4‐[(prop‐2‐en‐1‐yl)oxy]phenyl}propan‐2‐yl)phenol were the most toxic to HepG2 cells, and most of the other compounds inhibited cell proliferation. Moreover, typical compounds were also reproductive and developmental toxic to zebrafish embryos at different concentrations. The synthetic byproduct libraries could be used as pure standard compounds and applied in research on environmental behavior and the transformation of halogenated flame retardants.
中文翻译:

卤化双酚单取代醚的合成和毒性:为新兴污染物的潜在环境转化产物建立库
作为卤代双酚类化合物的一个重要分支,卤代双酚单取代醚类化合物因其毒性和变异性在环境健康科学中受到了广泛关注。在本研究中,开发了一种双酚单取代醚副产物库的合成方法。通过使用通用且高效的方法,四氯双酚 A、四溴双酚 A 和四溴双酚 S 单取代烷基醚化合物的产率为 39-82%。随后,使用三种不同的细胞系(HepG2、小鼠原代星形胶质细胞和 Chang 肝细胞)筛选了 27 种化合物的细胞毒性。化合物2,6-二溴-4-[3,5-二溴-4-(2-羟基乙氧基)苯-1-磺酰基]苯酚在各种细胞中比其他化合物毒性更大,并且该化合物对正常肝细胞和癌细胞的敏感性不一致。化合物 2,6-dichloro-4-(2-{3,5-dichloro-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 和 2,6- dibromo-4-(2-{3,5-dibromo-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 对 HepG2 细胞毒性最大,大部分其他化合物抑制细胞增殖。此外,典型的化合物在不同浓度下对斑马鱼胚胎也具有生殖和发育毒性。合成副产物库可用作纯标准化合物,可用于环境行为和卤化阻燃剂转化的研究。5-dibromo-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 对 HepG2 细胞的毒性最大,而大多数其他化合物抑制细胞增殖。此外,典型的化合物在不同浓度下对斑马鱼胚胎也具有生殖和发育毒性。合成副产物库可用作纯标准化合物,可用于环境行为和卤化阻燃剂转化的研究。5-dibromo-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 对 HepG2 细胞的毒性最大,而大多数其他化合物抑制细胞增殖。此外,典型的化合物在不同浓度下对斑马鱼胚胎也具有生殖和发育毒性。合成副产物库可用作纯标准化合物,可用于环境行为和卤化阻燃剂转化的研究。
更新日期:2020-10-16
中文翻译:

卤化双酚单取代醚的合成和毒性:为新兴污染物的潜在环境转化产物建立库
作为卤代双酚类化合物的一个重要分支,卤代双酚单取代醚类化合物因其毒性和变异性在环境健康科学中受到了广泛关注。在本研究中,开发了一种双酚单取代醚副产物库的合成方法。通过使用通用且高效的方法,四氯双酚 A、四溴双酚 A 和四溴双酚 S 单取代烷基醚化合物的产率为 39-82%。随后,使用三种不同的细胞系(HepG2、小鼠原代星形胶质细胞和 Chang 肝细胞)筛选了 27 种化合物的细胞毒性。化合物2,6-二溴-4-[3,5-二溴-4-(2-羟基乙氧基)苯-1-磺酰基]苯酚在各种细胞中比其他化合物毒性更大,并且该化合物对正常肝细胞和癌细胞的敏感性不一致。化合物 2,6-dichloro-4-(2-{3,5-dichloro-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 和 2,6- dibromo-4-(2-{3,5-dibromo-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 对 HepG2 细胞毒性最大,大部分其他化合物抑制细胞增殖。此外,典型的化合物在不同浓度下对斑马鱼胚胎也具有生殖和发育毒性。合成副产物库可用作纯标准化合物,可用于环境行为和卤化阻燃剂转化的研究。5-dibromo-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 对 HepG2 细胞的毒性最大,而大多数其他化合物抑制细胞增殖。此外,典型的化合物在不同浓度下对斑马鱼胚胎也具有生殖和发育毒性。合成副产物库可用作纯标准化合物,可用于环境行为和卤化阻燃剂转化的研究。5-dibromo-4-[(prop-2-en-1-yl)oxy]phenyl}propan-2-yl)phenol 对 HepG2 细胞的毒性最大,而大多数其他化合物抑制细胞增殖。此外,典型的化合物在不同浓度下对斑马鱼胚胎也具有生殖和发育毒性。合成副产物库可用作纯标准化合物,可用于环境行为和卤化阻燃剂转化的研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号