当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation of Nucleophilic Allylboranes from Molecular Hydrogen and Allenes Catalyzed by a Pyridonate Borane that Displays Frustrated Lewis Pair Reactivity.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-09-14 , DOI: 10.1002/anie.202011790 Max Hasenbeck 1 , Sebastian Ahles 1 , Arthur Averdunk 1 , Jonathan Becker 2 , Urs Gellrich 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-09-14 , DOI: 10.1002/anie.202011790 Max Hasenbeck 1 , Sebastian Ahles 1 , Arthur Averdunk 1 , Jonathan Becker 2 , Urs Gellrich 1
Affiliation
|
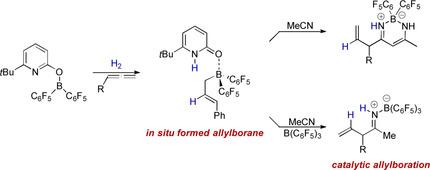
Here we report the in situ generation of nucleophilic allylboranes from H2 and allenes mediated by a pyridonate borane that displays frustrated‐Lewis‐pair reactivity. Experimental and computational mechanistic investigations reveal that upon H2 activation, the covalently bound pyridonate substituent becomes a datively bound pyridone ligand. Dissociation of the formed pyridone borane complex liberates Piers borane and enables a hydroboration of the allene. The allylboranes generated in this way are reactive towards nitriles. A catalytic protocol for the formation of allylboranes from H2 and allenes and the allylation of nitriles has been devised. This catalytic reaction is a conceptually new way to use molecular H2 in organic synthesis.
中文翻译:

由显示出沮丧的路易斯对反应性的吡啶酸酯硼烷催化的分子氢和丙二烯催化的亲核烯丙基硼烷的形成。
在这里,我们报道了由H 2和丙二烯介导的亲核烯丙基硼烷原位生成,其表现出沮丧的刘易斯对反应性。实验和计算机制研究表明,在H 2活化后,共价结合的吡啶酸酯取代基成为固定结合的吡啶酮配体。形成的吡啶酮硼烷络合物的离解释放了Piers硼烷,并使丙二烯进行硼氢化。以这种方式产生的烯丙基硼烷对腈具有反应性。已经设计了用于由H 2和烯丙基形成烯丙基硼烷和腈的烯丙基化的催化方案。从概念上讲,这种催化反应是使用分子H 2的新方法 在有机合成中。
更新日期:2020-09-14
中文翻译:

由显示出沮丧的路易斯对反应性的吡啶酸酯硼烷催化的分子氢和丙二烯催化的亲核烯丙基硼烷的形成。
在这里,我们报道了由H 2和丙二烯介导的亲核烯丙基硼烷原位生成,其表现出沮丧的刘易斯对反应性。实验和计算机制研究表明,在H 2活化后,共价结合的吡啶酸酯取代基成为固定结合的吡啶酮配体。形成的吡啶酮硼烷络合物的离解释放了Piers硼烷,并使丙二烯进行硼氢化。以这种方式产生的烯丙基硼烷对腈具有反应性。已经设计了用于由H 2和烯丙基形成烯丙基硼烷和腈的烯丙基化的催化方案。从概念上讲,这种催化反应是使用分子H 2的新方法 在有机合成中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号