Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-09-14 , DOI: 10.1016/j.jphotochem.2020.112905 Hiroshi Isobe , Mitsuo Shoji , Takayoshi Suzuki , Jian-Ren Shen , Kizashi Yamaguchi
|
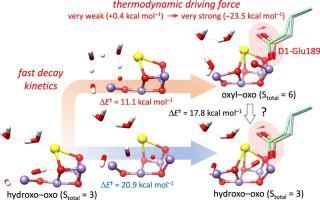
Photosynthetic oxidation of water to dioxygen is catalyzed by the Mn4CaO5 cluster in the protein-cofactor complex photosystem II. The light-driven catalytic cycle consists of four observable intermediates (S0, S1, S2, and S3) and one transient S4 state. Recently, using X-ray free-electron laser crystallography, two experimental groups independently observed incorporation of one additional oxygen into the cluster during the S2 to S3 transition, which is likely to represent a substrate. The present study implicates two competing reaction routes encountered during the structural rearrangement of the catalyst induced by the water binding and immediately preceding the formation of final stable forms in the S3 state. This mutually exclusive competition involves concerted versus stepwise conformational changes between two isomers, called open and closed cubane structures, which have different consequences on the immediate product in the S3 state. The concerted pathway involves a one-step conversion between two isomeric hydroxo forms without changes to the metal oxidation and total spin (Stotal = 3) states. Alternatively, in the stepwise process, the bound waters are oxidized and transformed into an oxyl–oxo form in a higher spin (Stotal = 6) state. Here, density functional calculations are used to characterize all relevant intermediates and transition structures and demonstrate that the stepwise pathway to the substrate activation is substantially favored over the concerted one, as evidenced by comparison of the activation barriers (11.1 and 20.9 kcal mol−1, respectively). Only after formation of the oxyl–oxo precursor can the hydroxo species be generated; this occurs with a slow kinetics and an activation barrier of 17.8 kcal mol−1. The overall thermodynamic driving force is likely to be controlled by the movements of two glutamate ligands, D1-Glu189 and CP43-Glu354, in the active site and ranges from very weak (+0.4 kcal mol−1) to very strong (–23.5 kcal mol−1).
中文翻译:

探索水结合在光系统II的氧演化复合物的S 3状态下由水结合引起的Mn团簇结构重排的反应途径
蛋白质-辅因子复合光系统II中的Mn 4 CaO 5团簇将水光合作用氧化为双氧。光驱动的催化循环由四个可观察到的中间体(S 0,S 1,S 2和S 3)和一个瞬态S 4状态组成。最近,使用X射线自由电子激光晶体学,两个实验组独立地观察到在S 2至S 3期间向团簇中引入了另外一种氧气过渡,这很可能代表基质。本研究暗示了由水结合引起的催化剂结构重排过程中,并且紧接在最终稳定形式的S 3状态形成之前遇到的两条竞争反应路线。这种相互排斥的竞争涉及到两个异构体之间的一致构象与逐步构象变化,这称为开放和封闭的古巴结构,这对S 3状态的直接产物具有不同的影响。一致的途径涉及两种异构羟基形式之间的一步转化,而金属氧化和总自旋(S total = 3)个状态。或者,在逐步过程中,结合的水被氧化并以较高的自旋(S total = 6)状态转化为oxyl-oxo形式。在这里,密度泛函计算用于表征所有相关的中间体和过渡结构,并证明与活化的基质相比,向基质活化的逐步途径明显偏爱协同活化的基质(通过比较活化壁垒(11.1和20.9 kcal mol -1,分别)。只有在形成羟基-氧代前体后,才能生成羟基。这会以缓慢的动力学和17.8 kcal mol -1的激活势垒发生。整个热力学驱动力很可能由两个谷氨酸配体D1-Glu189和CP43-Glu354在活动位点的移动控制,范围从非常弱(+0.4 kcal mol -1)到非常强(–23.5 kcal) mol -1)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号